Abstract
Purpose
To demonstrate the bioequivalence of the planned maleate salt-based commercial glasdegib tablet formulation [International Council for Harmonization (ICH) glasdegib] to the clinical di-hydrochloride (di-HCl) salt-based glasdegib formulation (di-HCl glasdegib). Additionally, to estimate the effects of a high-fat, high-calorie meal and proton-pump inhibitor (PPI) on the pharmacokinetics of ICH glasdegib.
Methods
This Phase I open-label study (ClinicalTrials.gov: NCT03130556) enrolled 24 healthy subjects to receive two different tablet formulations of single-dose 100-mg glasdegib under fasted conditions. A subset of healthy volunteers (n = 12) received single-dose 100-mg ICH glasdegib following a high-fat, high-calorie meal or concurrently with a PPI (rabeprazole).
Results
The adjusted geometric mean ratio (ICH glasdegib:di-HCl glasdegib) and 90% confidence intervals (CI) of area under the plasma concentration–time curve from time zero to infinity (AUCinf) and maximum plasma concentration (Cmax) were 104.0% (99.7‒108.5%) and 101.6% (96.1‒107.4%), respectively, within the acceptance range for bioequivalence (80‒125%). The adjusted geometric mean ratio (90% CIs) for AUCinf and Cmax under fed conditions were 84.3% (78.6‒90.6%) and 69.0% (61.8‒77.0%), respectively, relative to fasted conditions. When ICH glasdegib was administered concurrently with the PPI, the adjusted geometric mean ratio (90% CI) of AUCinf and Cmax were 100.6% (93.2‒108.6%) and 80.5% (70.7‒91.6%), respectively, relative to fasted conditions. Glasdegib was generally well tolerated under all conditions studied.
Conclusions
The ICH glasdegib tablet formulation was bioequivalent to the clinical di-HCl formulation under fasted conditions. A high-fat, high-calorie meal or concurrent PPI treatment had a minimal effect on glasdegib exposure, and was not considered clinically meaningful.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glasdegib (PF-04449913), an oral, small-molecule inhibitor of the smoothened receptor, selectively inhibits the Hedgehog signaling pathway [1]. Aberrant Hedgehog pathway signaling is associated with a variety of hematologic malignancies, and activation of the pathway is implicated in tumor formation, cancer progression, and drug resistance [2]. Preclinical studies have demonstrated glasdegib directly inhibits the growth of leukemic cells and is synergistic with chemotherapeutic agents [3, 4]. Glasdegib is currently in Phase III clinical development (ClinicalTrials.gov, NCT03416179) for the treatment of acute myeloid leukemia (AML); it is also being evaluated (ClinicalTrials.gov, NCT02367456) for high-risk myelodysplastic syndrome (MDS). Based on prior Phase I, single-agent, dose-escalation studies in patients with cancer, the recommended dose for glasdegib in patients with AML or MDS is 100 mg once daily (QD) [5].
Glasdegib is formulated as a 100-mg immediate-release oral tablet. Phase I/II clinical trials utilized a di-hydrochloride monohydrate (di-HCl glasdegib) formulation tablet, developed to determine the efficacy and safety of glasdegib in these early trials [6, 7]. However, the di-HCl glasdegib formulation was not considered optimal for further clinical development. During the drug development process, salt screening and optimization of the formulation are required to create a commercially viable tablet formulation. A previous study evaluated the use of physically stable, maleate salt-based glasdegib tablet formulations [8]. The study tested two novel maleate formulations with differing salt particle size, identifying them as bioequivalent to the di-HCl tablet formulation [8]. Additionally, the results of the study identified an acceptable range for active pharmaceutical ingredient particle size for future manufacture of maleate glasdegib tablet formulations. The proposed commercial maleate glasdegib tablet evaluated in the current report had changes to the drug load and changes in the percentage of some excipients, along with coloring and debossing.
Since glasdegib is orally administered, it is important to determine the pharmacokinetics (PK) in relation to meal consumption and concurrent medications, such as proton-pump inhibitors (PPIs), that might impact absorption. In vitro assessment determined the lipophilicity of glasdegib to be low (cLogP = 2.28), with glasdegib considered to be a Biopharmaceutical Classification System class II or IV drug (unpublished) [1]. The effect of a high-fat, high-calorie meal on the PK of the di-HCl formulation tablet and earlier maleate-salt formulations was previously investigated [8, 9]. Based on these studies, the recommendation was that glasdegib may be taken independent of food intake. The solubility of glasdegib, a weakly basic drug, is hydrogen (pH)-dependent, with decreasing solubility as pH increases. Therefore, glasdegib PK could theoretically be altered when concomitantly administered with drugs that elevate gastric pH, such as PPIs. Cancer patients frequently receive PPIs for the treatment or prophylaxis of concomitant conditions; understanding the potential effects of these drugs on the bioavailability of glasdegib is therefore important [10]. The effects of PPIs on the PK of the early maleate-salt glasdegib tablet formulations were previously evaluated [8]. Glasdegib dosing in the presence of PPIs resulted in a 12% increase in area under the plasma concentration–time curve (AUC) from time zero to infinity (AUCinf) and a 13% decrease in maximum plasma concentration (Cmax), indicating no clinically relevant effect of PPIs on glasdegib following a single 100-mg oral dose. Therefore, the current study aimed to estimate the impact of food and a PPI on the planned commercial formulation of glasdegib.
The present study evaluated a maleate salt formulation of glasdegib that is compliant with the International Council for Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH glasdegib) [11]. This formulation is intended to be the final commercial formulation. The primary objective of this study was to establish bioequivalence of the new ICH glasdegib tablet formulation to the di-HCl glasdegib formulation. The secondary aims were to determine the effects of a high-calorie, high-fat meal or a PPI (rabeprazole) on the plasma PK of single-dose ICH glasdegib. Additionally, the safety and tolerability of a single dose of glasdegib administered in fasted or fed states or in the presence of a PPI were evaluated. This study was not designed, nor did it aim to demonstrate BE or test for BE between fasted di-HCL and fed ICH maleate glasdegib.
Materials and methods
Study design
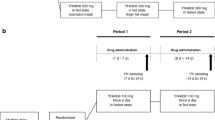
This was a Phase I, randomized, open-label, three-period, four-sequence, four-treatment study in healthy volunteers. Subjects were randomized to one of four treatment sequences (Fig. 1). All subjects received three treatments with a washout period of at least 7 days between each administration period. The duration of each administration period was 6 days with the exception of PPI treatment, which was 12 days (including 6-day follow-up after glasdegib administration). Glasdegib was administered with 240 mL of water, and water was withheld for 1 h both before and after administration.
Treatment sequence. Healthy volunteers received 100 mg of glasdegib as a single, oral, instant-release formulation tablet. All doses were given in a fasted state (> 10 h) unless otherwise stated. The duration of each period was 6 days following glasdegib administration. N number of subjects, PPI proton-pump inhibitor (rabeprazole)
The primary study objective was to determine the bioequivalence of the new ICH glasdegib formulation to the di-HCl glasdegib formulation (determined in periods 1 and 2) using a two-way crossover study design. Subjects received a 100-mg single dose of di-HCl glasdegib or ICH glasdegib, which was administered after an overnight fast of at least 10 h. No food was allowed for at least 4 h after glasdegib dosing.
The effects of food on the PK of ICH glasdegib were determined during period 3 in a subset of subjects (n = 12) using a one-way crossover study design. Following a 10-h fast, subjects consumed a recommended high-fat (~ 50% of total caloric content of the meal), high-calorie meal (~ 800 to 1000 calories with 150, 250, and 500–600 calories from protein, carbohydrate, and fat, respectively). Subjects received a 100-mg single dose of ICH glasdegib ~ 5 min after the meal was consumed. The reference for this evaluation was ICH glasdegib administered under fasted conditions during periods 1 and 2.
The effect of a PPI (rabeprazole) on the PK of ICH glasdegib was determined during period 3 in a subset of subjects (n = 12) using a one-way crossover study design. Following a 7-day washout period, rabeprazole 40 mg was administered daily for 7 days. On the day of PK sample collection (day 7 of rabeprazole administration), both rabeprazole and glasdegib were administered in the fasted state. Rabeprazole was administered 4 h before glasdegib. No food was allowed for 4 h following glasdegib administration. The reference for this objective was ICH glasdegib administered under fasted conditions (periods 1 and 2).
The primary PK endpoints were AUCinf and Cmax of glasdegib. Additional PK endpoints were AUC from time 0 to the last quantifiable concentration (AUClast), time for Cmax (Tmax), plasma elimination half-life (t½), apparent oral clearance (CL/F), and apparent volume of distribution following oral dose (Vz/F).
Subjects
Eligible subjects were healthy females of non-childbearing potential and males 18‒55 years of age, with a body mass index (BMI) of 17.5‒30.5 kg/m2. Subjects were in good health based on medical history, physical examination (including blood pressure and pulse rate measurements), 12-lead electrocardiogram (ECG), and clinical laboratory tests. Subjects were excluded if they did not meet entry criteria, including if they had a known sensitivity to PPIs, donated blood within 60 days prior to dosing, had any condition possibly affecting drug absorption (e.g., gastrectomy, achlorhydria) or used prescription/non-prescription drugs and dietary supplements within 7 days or five half-lives [whichever was longer (with the exception of acetaminophen/paracetamol)], or had abnormal clinical laboratory tests for liver function. Subjects were also excluded if they had an ECG demonstrating the time from the beginning of the Q wave to the end of the T wave corresponding to electrical systole (QT) corrected for the heart rate interval > 450 ms or a time from ECG Q wave to the end of the S wave corresponding to ventricle depolarization (QRS) interval > 120 ms or had a family history of myocardial infarction, congenital long QT syndrome, torsades de pointes, or clinically significant ventricular arrhythmias.
Ethical approval
The study protocol was approved by an independent institutional review board and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Informed consent was obtained from all individual participants included in the study. This trial is registered with ClinicalTrials.gov, identifier NCT03130556.
Pharmacokinetic assessments and analysis
Blood samples for PK analysis were collected at 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 10, 24, 48, 72, 96, and 120 h following glasdegib administration, using dipotassium ethylenediaminetetraacetic acid tubes. Glasdegib plasma concentrations were determined at Covance Bioanalytical Services (Shanghai, China) using a validated, sensitive, and specific high-performance liquid chromatography–tandem mass spectrometric (HPLC–MS/MS) method. A 50-µL plasma aliquot was spiked with deuterated internal standard (glasdegib-d4), followed by addition of 10% NH4OH (aq), extraction with 1000 µL ethyl acetate, and centrifugation. A 400-µL aliquot of the organic layer was evaporated to dryness under a stream of nitrogen, and the residue was reconstituted with 400 µL of 0.1% formic acid in acetonitrile:water (25:75 v/v) and injected into the HPLC–MS/MS system. Chromatographic separation was achieved with a Zorbax XDB-C18 (50 × 2.1 mm, 5 µm; Agilent Technologies, Santa Clara, CA) HPLC column heated to 30 °C and a mobile-phase gradient at a flow rate of 600 µL/min. Mobile phase A consisted of 0.1% formic acid in water and mobile phase B consisted of 0.1% formic acid in acetonitrile. The mobile-phase composition started at 20% B for 0.4 min and increased linearly to 75% B over 1.6 min. Detection of glasdegib and the internal standard was by MS/MS (Sciex API 4000; Applied Biosystems, Foster City, CA) in multiple reaction monitoring mode using positive ion electrospray (IonSpray voltage of 3000 V and temperature at 550 °C). The monitored ion transitions were m/z 375 → 257 for glasdegib and m/z 379 → 257 for the internal standard.
Calibration curves were linear over the range of 3–3000 ng/mL for glasdegib in plasma, using a weighted (1/concentration2) linear regression. The lower limit of quantification (LLOQ) of glasdegib was 3 ng/mL. PK plasma samples were stored at − 70 °C and assayed within the 575 days of established frozen plasma stability. Inter-assay accuracy (percent relative error) at 9, 100, and 2250 ng/mL glasdegib in quality control samples ranged from − 0.4 to 2.0%. Inter-assay precision [percent coefficient of variation (%CV)] was ≤ 6.1% across quality control levels.
Glasdegib PK parameters were calculated using noncompartmental analysis of plasma concentration–time data. Samples below LLOQ were set to 0 for analysis. Actual sample collection times were used for the PK analysis. Cmax and Tmax were the observed values. AUClast was determined using the linear/log trapezoidal method. AUCinf was calculated as AUClast + (Clast/Kel), where Clast was the predicted plasma concentration at the last quantifiable time point estimated from the log-linear regression analysis and Kel was the terminal phase rate constant calculated by a linear regression of the log-linear concentration-time curve. The t½ was calculated as loge(2)/Kel, CL/F was calculated as dose/AUCinf, and Vz/F was calculated as dose/(AUCinf × Kel).
Safety assessments
All subjects received glasdegib treatment and were included in the safety analyses. Safety and tolerability of glasdegib were assessed by adverse event (AE) monitoring and changes in clinical laboratory results, ECGs, and physical examination findings. AEs were graded according to the Medical Dictionary for Regulatory Activities version 20.0.
Sample size determination
For bioequivalence, a sample size of 24 subjects provided (i) 98% power, so that the 90% confidence interval (CI) for the ratio of test-to-reference treatment for glasdegib AUCinf would be within the acceptance range of 80‒125% and (ii) 92% power, so that the 90% CI for the ratio of test-to-reference treatment for Cmax would be within the acceptance range of 80‒125%. This estimate was based on the assumption that the true ratio of test-to-reference for both AUCinf and Cmax was 1.05, within-subject standard deviations of 0.144 and 0.179 for loge AUCinf and logeCmax, respectively, obtained from the mean of three prior studies of glasdegib [8, 9, 12].
The sample size of 12 subjects per group for assessment of the effect of food or a PPI on glasdegib PK was chosen empirically, as these assessments were for estimation purposes. A sample size of 12 subjects provided 90% CIs for the difference between treatments of ± 0.1323 and ± 0.1645 on the natural log scale for AUCinf and Cmax, respectively, with 90% coverage probability.
Statistical analysis
To determine the bioequivalence of ICH glasdegib to di-HCl glasdegib, natural log-transformed AUCinf, AUClast, and Cmax for glasdegib were analyzed using a mixed-effects model with sequence, period, and treatment as fixed effects and subject within sequence as a random effect. To estimate the effects of food and PPIs on the bioavailability of glasdegib, natural log-transformed AUCinf, AUClast, and Cmax for glasdegib were analyzed using a mixed-effects model with treatment as a fixed effect and subject as a random effect. Adjusted mean differences and 90% CIs for the differences from the models were exponentiated to provide estimates of the adjusted geometric mean ratio and 90% CIs (Test:Reference).
Results
Subjects and baseline characteristics
A total of 24 subjects were enrolled in the study, and six subjects were randomized to each of the four sequences. All enrolled subjects received glasdegib treatment and completed the study. The mean age of subjects was 37 years (range 25‒53 years) and the majority of subjects were black (n = 17). All but one subject was male. The mean weight was 82.7 kg (range 58.5‒95.7 kg), with a mean height of 175.7 cm (range 165‒189 cm). The mean BMI was 26.8 kg/m2 (range 19.3‒30.5 kg/m2).
Pharmacokinetic results
Bioequivalence of ICH glasdegib
The PK parameters for ICH glasdegib and di-HCl glasdegib under fasted conditions are summarized in Table 1. The median plasma glasdegib concentration–time profiles for both formulations are presented in Fig. 2a. The ratios (ICH glasdegib:di-HCl glasdegib) of adjusted geometric means of glasdegib AUCinf and Cmax were 104.0% (90% CI 99.7‒108.5%) and 101.6% (90% CI 96.1‒107.4%), respectively (Table 1). The corresponding 90% CIs for the ratios of adjusted geometric means were contained within the acceptance range for bioequivalence (80‒125%). The median (range) Tmax was 1.0 (0.5–3.0) h for di-HCl glasdegib and 2.0 (0.5–4.0) h for ICH glasdegib. The apparent terminal t½ values were similar for the two treatments, with mean values of 14.99 h and 15.20 h for di-HCl glasdegib and ICH glasdegib, respectively. Inter-subject variability for AUCinf and Cmax for the two formulations was similar, with geometric %CV ranging 30–31% for AUCinf and 28–30% for Cmax. No sequence or period effects were observed for Cmax, AUClast, and AUCinf.
Effect of food
The median plasma concentration–time profiles for a single dose of ICH glasdegib administered following either overnight fasting (fasted) or after a high-fat, high-calorie meal (fed) are presented in Fig. 3a. Changes in AUCinf and Cmax due to food effect for each subject are provided in Fig. 3b, c. The PK parameters for ICH glasdegib under fed conditions are summarized in Table 1. The ratios [ICH glasdegib (fed):ICH glasdegib (fasted)] of adjusted geometric mean ratio (90% CIs) of AUCinf and Cmax were 84.3% (78.6‒90.6%) and 69.0% (61.8‒77.0%), respectively (Table 1). The observed median Tmax (range) post-dose was 3.0 (1.0–6.0) h under fed conditions and 2.0 (0.5–4.0) h under fasted conditions. The mean apparent terminal t½ was similar under both conditions (16.08 vs. 15.20 h, ICH glasdegib fed vs. fasted). Inter-subject variability for glasdegib exposure based on geometric %CV ranged from 24 to 30% for AUCinf and from 30 to 33% for Cmax for the effect of food on treatment.
Effect of food on ICH glasdegib. a Linear median glasdegib plasma concentration–time profiles for glasdegib given under fasted and fed conditions (inset contains same data as semi-log profile), b Matchstick plots for change in exposure for each subject when ICH glasdegib is given under fasted and fed conditions for AUCinf, and cCmax
Effect of a PPI
The median plasma glasdegib concentration–time profiles for subjects who received or did not receive a PPI (rabeprazole) are presented in Fig. 4a. Changes in AUCinf and Cmax due to PPI effect for each subject are provided in Fig. 4b, c. PK parameters for ICH glasdegib administered with a PPI are summarized in Table 1. The ratios [ICH glasdegib + PPI:ICH glasdegib (fasted)] of adjusted geometric means (90% CIs) of AUCinf and Cmax were 100.6% (93.2‒108.6%) and 80.5% (70.7‒91.6%), respectively (Table 1). The median Tmax (range) post-dose was 3.0 (1.5–6.0) h with PPI treatment, compared with 2.0 (0.5–4.0) h for ICH glasdegib alone. The mean apparent terminal t½ was similar under both treatments (15.39 vs. 15.20 h, ICH glasdegib with vs. without PPI). Inter-subject variability for glasdegib exposure based on geometric %CV ranged from 30 to 42% for AUCinf and from 27 to 30% for Cmax for PPI effect on treatment.
Effect of PPI (rabeprazole) on ICH glasdegib. a Linear median glasdegib plasma concentration–time profiles for glasdegib given alone and with a PPI (inset contains same data as semi-log profile), b Matchstick plots for change in exposure for each subject when ICH glasdegib is given with and without a PPI for AUCinf, and cCmax
Safety
A single 100-mg dose of glasdegib was generally well tolerated when administered under fasted or fed conditions and with a PPI in healthy subjects. The incidence of treatment-emergent AEs (TEAEs) is summarized in Table 2. Overall, 17 TEAEs were reported in 12 subjects across all treatment arms [di-HCl glasdegib, n = 5; ICH glasdegib, n = 2; ICH glasdegib (fed), n = 3; ICH glasdegib + PPI, n = 2]. The most commonly observed TEAEs were gastrointestinal disorders (35% of all TEAEs), particularly vomiting. All observed TEAEs were mild, and none were determined by the investigator to be treatment-related. All TEAEs had resolved by the end of the study. There were no deaths, serious AEs, severe AEs, dose reductions, or permanent/temporary discontinuations due to AEs reported in this study.
Discussion
A new maleate-salt formulation glasdegib tablet that is compliant with the ICH guidelines was developed following an earlier study that determined an active pharmaceutical ingredient particle size for the manufacture of maleate glasdegib tablet formulations [8]. The primary goal of this study was to determine the bioequivalence of the new ICH glasdegib formulation to the di-HCl formulation used in previous Phase I/II trials, including the Phase II clinical trial that generated efficacy and safety data used in glasdegib filings for regulatory approval for treatment of patients with AML or MDS [13]. In these early clinical trials, glasdegib was initially administered in the fasted state, with no food allowed 2 h before and 2 h after the daily dose, and eventually tested irrespective of food consumption [7, 12, 13]. The new tablet formulation is physically more stable than the previously tested di-HCl glasdegib tablet and is intended to be the final commercial formulation. This study determined the 100-mg ICH maleate glasdegib formulation to be bioequivalent to the 100-mg di-HCl tablet formulation under fasted conditions because the 90% CI for the ratios (ICH glasdegib/di-HCl glasdegib) of the adjusted geometric mean ratio fell wholly within the acceptance interval of 80–125% for both AUCinf and Cmax.
This study was designed to estimate the maximal effect of food on the PK of oral ICH glasdegib by evaluating the effect of a high-fat, high-calorie meal consumption immediately prior to glasdegib administration [14]. Food ingestion can change the bioavailability of oral medications by various means, including delaying gastric emptying, stimulation of bile flow, changes in gastrointestinal pH, increase of splanchnic blood flow, changes in luminal metabolism of the studied drug, or physical or chemical food interactions with the drug product [14]. Administration of ICH glasdegib in the presence of a high-fat, high-calorie meal resulted in a 16% decrease in geometric mean AUCinf and 31% decrease in geometric mean Cmax compared with administration under fasted conditions (Table 1; Fig. 3a). The change in both AUC inf and Cmax in individual subjects was relatively consistent (Fig. 3b, c). There was a minimal difference in the median Tmax in the presence of food, wherein the range of Tmax was comparable with and without food. These results are similar to those previously reported for the effect of food on the di-HCl tablet formulation and a previous maleate tablet formulation [8, 9]. Based on preclinical studies, the efficacy of glasdegib is considered to be due to the continuous inhibition of the Hedgehog pathway. Therefore, small changes in overall exposure (AUCinf) are likely not clinically significant [9]. Inhibition of the Hedgehog pathway in skin has typically been used to measure the pharmacodynamics of smoothened inhibitors. With glasdegib, consistent downregulation of the Hedgehog pathway has been observed at the 50 mg QD dose, indicating the modulation of pathway could be maintained in the scenario of lowered exposure due to food [15].
Additionally, in the first-in-patient study in patients with hematologic malignancies, signs of clinical activity were noted over a wide range of dose levels tested [5]. The clinical efficacy of the clinical dose of 100 mg QD was further established in a randomized Phase II study in patients with AML or high-risk MDS dosed with glasdegib without regard to food and use of pH altering agents that demonstrated survival benefit when in combination with chemotherapy compared with chemotherapy alone [16]. Therefore, food appeared to have a minimal effect on glasdegib exposure (16% reduction in AUCinf) following a single oral dose, and its impact on the PK of glasdegib was not considered clinically meaningful.
The solubility of glasdegib, a weakly basic drug, is pH-dependent, with higher solubility at acidic pH that drops substantially at pH ≥ 6.3. Therefore, PPIs may affect the solubility of co-administered drugs by elevating gastric pH, potentially resulting in reduced bioavailability. Healthy volunteers received a PPI (rabeprazole) over a 7-day period prior to dosing with glasdegib, ensuring an elevated gastric pH at the time of glasdegib administration [17]. Administration of glasdegib in the presence of a PPI did not result in a change in geometric mean AUCinf, whereas there was a decrease (~ 20%) in geometric mean Cmax compared with administration under fasted conditions (Table 1; Fig. 4a). The change in both AUCinf and Cmax in individual subjects was inconsistent (Fig. 4b, c), with increases, decreases, and no change observed in different subjects. There was minimal difference in the median Tmax in the presence of PPI compared with the half-life of glasdegib, wherein the range of Tmax was approximately similar with vs. without PPI. These findings are similar to results previously reported for the effect of PPI on the tablet formulation [8]. Overall, treatment with a PPI had no clinically meaningful effect (AUCinf, 100.6%) on the plasma PK of 100-mg ICH glasdegib.
A single oral dose of glasdegib was well tolerated, and all AEs observed were mild in fasted or fed states and not considered treatment-related in healthy subjects. The most frequently reported AEs were gastrointestinal disorders (diarrhea, vomiting, and nausea) that were resolved by the end of the study.
In conclusion, 100-mg ICH glasdegib formulation tablet, tested under fasted conditions, is bioequivalent to the clinical 100-mg di-HCl glasdegib formulation used in the study that generated efficacy and safety data used for regulatory submission. The results from this clinical study in healthy volunteers demonstrated that food and agents that increase gastric pH (rabeprazole) had a minimal effect on glasdegib exposure, and therefore not considered to be clinically relevant. Additionally, ICH glasdegib was generally safe and well tolerated under all tested conditions. Therefore, ICH glasdegib may be taken irrespective of food intake and administration of pH-increasing agents, which simplifies dosing recommendations and may facilitate compliance in patients with cancer.
References
Munchhof MJ, Li Q, Shavnya A, Borzillo GV, Boyden TL, Jones CS, LaGreca SD, Martinez-Alsina L, Patel N, Pelletier K et al (2012) Discovery of PF-04449913, a potent and orally bioavailable inhibitor of Smoothened. ACS Med Chem Lett 3:106–111. https://doi.org/10.1021/ml2002423
Campbell V, Copland M (2015) Hedgehog signaling in cancer stem cells: a focus on hematological cancers. Stem Cells Cloning 8:27–38. https://doi.org/10.2147/SCCAA.S58613
Sadarangani A, Pineda G, Lennon KM, Chun HJ, Shih A, Schairer AE, Court AC, Goff DJ, Prashad SL, Geron I et al (2015) GLI2 inhibition abrogates human leukemia stem cell dormancy. J Transl Med 13:98. https://doi.org/10.1186/s12967-015-0453-9
Fukushima N, Minami Y, Kakiuchi S, Kuwatsuka Y, Hayakawa F, Jamieson C, Kiyoi H, Naoe T (2016) Small-molecule Hedgehog inhibitor attenuates the leukemia-initiation potential of acute myeloid leukemia cells. Cancer Sci 107:1422–1429. https://doi.org/10.1111/cas.13019
Martinelli G, Oehler VG, Papayannidis C, Courtney R, Shaik MN, Zhang X, O’Connell A, McLachlan KR, Zheng X, Radich J et al (2015) Treatment with PF-04449913, an oral smoothened antagonist, in patients with myeloid malignancies: a phase 1 safety and pharmacokinetics study. Lancet Haematol 2:e339–e346. https://doi.org/10.1016/S2352-3026(15)00096-4
Wagner AJ, Messersmith WA, Shaik MN, Li S, Zheng X, McLachlan KR, Cesari R, Courtney R, Levin WJ, El-Khoueiry AB (2015) A phase I study of PF-04449913, an oral hedgehog inhibitor, in patients with advanced solid tumors. Clin Cancer Res 21:1044–1051. https://doi.org/10.1158/1078-0432.CCR-14-1116
Lam JL, Vaz A, Hee B, Liang Y, Yang X, Shaik MN (2017) Metabolism, excretion and pharmacokinetics of [(14)C]glasdegib (PF-04449913) in healthy volunteers following oral administration. Xenobiotica 47:1064–1076. https://doi.org/10.1080/00498254.2016.1261307
Giri N, Lam LH, LaBadie RR, Krzyzaniak JF, Jiang H, Hee B, Liang Y, Shaik MN (2017) Evaluation of the effect of new formulation, food, or a proton pump inhibitor on the relative bioavailability of the Smoothened inhibitor glasdegib (PF-04449913) in healthy volunteers. Cancer Chemother Pharmacol 80:1249–1260. https://doi.org/10.1007/s00280-017-3472-9
Shaik MN, LaBadie RR, Rudin D, Levin WJ (2014) Evaluation of the effect of food and ketoconazole on the pharmacokinetics of the Smoothened inhibitor PF-04449913 in healthy volunteers. Cancer Chemother Pharmacol 74:411–418. https://doi.org/10.1007/s00280-014-2502-0
Numico G, Fusco V, Franco P, Roila F (2017) Proton pump inhibitors in cancer patients: How useful they are? A review of the most common indications for their use. Crit Rev Oncol Hematol 111:144–151. https://doi.org/10.1016/j.critrevonc.2017.01.014
International Council for Harmonisation (ICH) (2018) ICH guidelines. http://www.ich.org/products/guidelines.html. Accessed 27 Aug 2018
Shaik MN, Hee B, Wei H, LaBadie RR (2018) Evaluation of the effect of rifampin on the pharmacokinetics of the Smoothened inhibitor glasdegib in healthy volunteers. Br J Clin Pharmacol. https://doi.org/10.1111/bcp.13568
Cortes JE, Smith BD, Wang ES, Merchant A, Oehler VG, Arellano M, DeAngelo DJ, Pollyea DA, Sekeres MA, Robak T et al (2018) Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: phase 2 study results. Am J Hematol. https://doi.org/10.1002/ajh.25238
US Food and Drug Administration (2002) Guidance for industry. Food-effect bioavailability and fed bioequivalence studies (last update: December 2002). https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070241.pdf. Accessed 27 Aug 2018
Minami Y, Minami H, Miyamoto T, Yoshimoto G, Kobayashi Y, Munakata W, Onishi Y, Kobayashi M, Ikuta M, Chan G et al (2017) Phase I study of glasdegib (PF-04449913), an oral smoothened inhibitor, in Japanese patients with select hematologic malignancies. Cancer Sci 108:1628–1633. https://doi.org/10.1111/cas.13285
Cortes JE, Heidel FH, Heuser M, Fiedler W, Smith BD, Robak T, Montesinos Fernandez P, Ma WW, Shaik MN, Zeremski M et al (2016) A phase 2 randomized study of low dose Ara-C with or without glasdegib (PF-04449913) in untreated patients with acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood 128:99
Williams MP, Blanshard C, Millson C, Sercombe J, Pounder RE (2000) A placebo-controlled study to assess the effects of 7-day dosing with 10, 20 and 40 mg rabeprazole on 24-h intragastric acidity and plasma gastrin in healthy male subjects. Aliment Pharmacol Ther 14:691–699
Acknowledgements
This study was sponsored by Pfizer and was conducted at the Pfizer Clinical Research Unit in New Haven, CT. Medical writing support was provided by Gemma Shay, PhD, at Engage Scientific Solutions and was funded by Pfizer.
Funding
This study was sponsored by Pfizer and was conducted at the Pfizer Clinical Research Unit in New Haven, CT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
B. Hee, H. Wei, R. R. LaBadie, and N. Shaik are employees of Pfizer and own Pfizer stock and options.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Data sharing statement
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shaik, N., Hee, B., Wei, H. et al. Evaluation of the effects of formulation, food, or a proton-pump inhibitor on the pharmacokinetics of glasdegib (PF-04449913) in healthy volunteers: a randomized phase I study. Cancer Chemother Pharmacol 83, 463–472 (2019). https://doi.org/10.1007/s00280-018-3748-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3748-8