Abstract
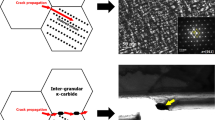
The cyclic oxidation behavior of HK30Nb heat-resistant steel processed by laser powder bed fusion (LPBF) was compared to its cast counterpart during exposures in air and air + 10% H2O at 800 °C. The specific finer microstructure and lower Mn of the LPBF alloy resulted in lower oxidation rates in dry air and faster establishment of a continuous Cr2O3 scale in air + 10% H2O compared to coarse-grained cast HK30Nb with higher Mn. Differences in alloy mechanical strength and therefore their ability to accommodate high temperature and oxidation-induced stresses as well as differences in thermal expansion coefficients between the alloy and the formed oxides (Cr2O3 only for the LPBF and Cr2O3 and MnCr2O4 for the cast specimens) during temperature cycling were found to result in a greater extent of spallation for the LPBF than for the cast alloy in dry air at 800 °C.
















Similar content being viewed by others
References
A. S. Sabau, et al., Design, additive manufacturing, and performance of heat exchanger with a novel flow-path architecture. Applied Thermal Engineering 180, 115775 (2020).
S. Dryepondt, et al., High Temperature High Strength Austenitic Steel Fabricated by Laser Powder-Bed Fusion. SSRN Electronic Journal, 2021.
T. DebRoy, et al., Additive manufacturing of metallic components: Process, structure and properties. Progress in Materials Science 92, 112–224 (2018).
L. Liu, et al., Dislocation network in additive manufactured steel breaks strength–ductility trade-off. Materials Today 21, (4), 354–361 (2018).
Dryepondt, S. N., et al., Microstructure and high temperature tensile properties of 316L fabricated by laser powder-bed fusion. 37 (2020).
D. Kong, et al., About metastable cellular structure in additively manufactured austenitic stainless steels. Additive Manufacturing 38, 101804 (2021).
T. Voisin, et al., New insights on cellular structures strengthening mechanisms and thermal stability of an austenitic stainless steel fabricated by laser powder-bed-fusion. Acta Materialia 203, 116476 (2021).
Y. M. Wang, et al., Additively manufactured hierarchical stainless steels with high strength and ductility. Nature Materials 17, (1), 63–71 (2018).
M. P. Brady, et al., Long-Term Oxidation of Candidate Cast Iron and Stainless Steel Exhaust System Alloys from 650 to 800 °C in Air with Water Vapor. Oxidation of Metals 82, (5), 359–381 (2014).
D. J. Young, Oxidation behaviour of some modern heat-resistant cast steels. High Temperature Technology 1, (2), 101–105 (1982).
M. Schütze, Fundamentals of High Temperature Corrosion. In Materials Science and Technology: A Comprehensive Treatment (2000), p. 67–130.
P. Ramos, et al., Residual stress analysis in thermally grown oxide scales developed on Nb-alloyed refractory austenitic stainless steels. Corrosion Science 178, 109066 (2021).
T. Sanviemvongsak, D. Monceau, and B. Macquaire, High temperature oxidation of IN 718 manufactured by Laser Beam Melting and Electron Beam Melting: Effect of surface topography. Corrosion Science 141, 127 (2018).
T. Sanviemvongsak, et al., Intergranular Oxidation of Ni-base Alloy 718 with a focus on Additive Manufacturing. Corrosion Science 170, 108684 (2020).
M. Romedenne, et al., High temperature air oxidation behavior of Hastelloy X processed by Electron Beam Melting (EBM) and Selective Laser Melting (SLM). Corrosion Science 171, 108647 (2020).
M. C. Kuner, et al., Quantitatively accounting for the effects of surface topography on the oxidation kinetics of additive manufactured Hastelloy X processed by electron beam melting. Additive Manufacturing 36, 101431 (2020).
N. Ramenatte, et al., A comparison of the high-temperature oxidation behaviour of conventional wrought and laser beam melted Inconel 625. Corrosion Science 164, 108347 (2020).
A. Casadebaigt, J. Hugues, and D. Monceau, Influence of Microstructure and Surface Roughness on Oxidation Kinetics at 500–600 °C of Ti–6Al–4V Alloy Fabricated by Additive Manufacturing. Oxidation of Metals 90, 633 (2018).
C. Siri, et al., Impact of Selective Laser Melting Additive Manufacturing on the High Temperature Behavior of AISI 316L Austenitic Stainless Steel. Oxidation of Metals 94, (5), 527–548 (2020).
Siri, C., et al., Impact of Water Vapor on the High Temperature Oxidation of Wrought and Selective Laser Melted (SLM) AISI 316L. Oxidation of Metals 1–13 (2021).
T. Sanviemvongsak, et al., Cyclic oxidation of alloy 718 produced by additive manufacturing compared to a wrought-718 alloy. Corrosion Science 192, 109804 (2021).
M. Romedenne, P. Stack, R. Pillai, and S. Dryepondt, Isothermal and cyclic oxidation of Haynes 282 processed by Electron Beam Melting (EBM) and Laser Powder Bed Fusion (LPBF) in dry air at 800 and 950°C. JOM 74, 1–12 (2022).
Dryepondt, S., M.M. Kirka, and F.A. List, III, Oxidation Behavior of Ni-Based Alloys Fabricated by Additive Manufacturing, in NACE 2019.
Armstrong, J.T., Quantitative analysis of silicate and oxide materials: Comparison of Monte Carlo, ZAF and phi-rho-z procedures. Microbeam Analysis ed. 1988, San Francisco, California, USA: San Francisco Press.
S. Dryepondt, et al., High temperature high strength austenitic steel fabricated by laser powder-bed fusion. Acta Materialia 231, 117876 (2022).
Pillai, R., S. Dryepondt, and B.A. Pint. High Temperature Oxidation Lifetime Modeling of Thin-Walled Components. in ASME Turbo Expo 2019: Turbomachinery Technical Conference and Exposition. 2019.
C. E. Lowell, et al., COSP: a Computer-Model of Cyclic Oxidation. Oxidation of Metals 36, (1–2), 81–112 (1991).
J. Zurek, et al., Growth and adherence of chromia based surface scales on Ni-base alloys in high- and low-pO(2) gases. Materials Science and Engineering A 477, (1–2), 259–270 (2008).
T. Perez, et al., About the Synergetic Influence of Manganese and Silicon on the Oxidation Rate of Chromia Forming Nickel-Based Model Alloys at 1050 °C. Oxidation of Metals 94, (3), 235–249 (2020).
A. Petric and H. Ling, Electrical Conductivity and Thermal Expansion of Spinels at Elevated Temperatures. Journal of the American Ceramic Society 90, (5), 1515–1520 (2007).
H. E. Evans, Stress Effects in High-Temperature Oxidation of Metals. International Materials Reviews 40, (1), 1–40 (1995).
P. Huczkowski, et al., Effect of component thickness on lifetime and oxidation rate of chromia forming ferritic steels in low and high pO(2) environments. Materials at High Temperatures 22, (3–4), 253–262 (2005).
R. Duan, et al., Predicting Oxidation-Limited Lifetime of Thin-Walled Components of NiCrW Alloy 230. Oxidation of Metals 87, (1), 11–38 (2017).
J. L. Smialek, Oxidation Resistance and Critical Sulfur Content of Single Crystal Superalloys. Journal of Engineering for Gas Turbines and Power 120, (2), 370–374 (1998).
J. Smialek, Origins of a Low-Sulfur Superalloy Al 2 O 3 Scale Adhesion Map. Crystals 2021, 60 (2021).
P. Y. Hou and J. Stringer, Oxide scale adhesion and impurity segregation at the scale/metal interface. Oxidation of Metals 38, (5), 323–345 (1992).
G. R. Holcomb, Calculation of reactive-evaporation rates of chromia. Oxidation of Metals 69, (3–4), 163–180 (2008).
D. J. Young and B. A. Pint, Chromium volatilization rates from Cr2O3 scales into flowing gases containing water vapor. Oxidation of Metals 66, (3–4), 137–153 (2006).
W. J. Quadakkers, J. Zurek, and M. Hänsel, Effect of water vapor on high-temperature oxidation of FeCr alloys. JOM 61, (7), 44–50 (2009).
B. A. Pint, Addressing the Role of Water Vapor on Long-Term Stainless Steel Oxidation Behavior. Oxidation of Metals 95, (5–6), 335–357 (2021).
Acknowledgements
The authors would like to thank M. Stephens, J. Wade, T. Lowe, V. Cox, C. O’Dell and E. Cakmak for their assistance with the experimental work. M. Ridley and P. Fernandez-Zelaia are kindly acknowledged for their detailed comments on the manuscript. This research was sponsored by the US Department of Energy, Office of Energy Efficiency and Renewable Energy (EERE), Vehicle Technologies Office, Propulsion Materials Program, and the EERE Advanced Manufacturing Office, Combined Heat and Power Program under contract DE-AC05-00OR22725 with UT-Battelle LLC. The work was performed in partiality at the Oak Ridge National Laboratory’s Manufacturing Demonstration Facility, an Office of Energy Efficiency and Renewable Energy user facility. The authors declare no conflict of interest. Notice: This manuscript has been authored by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). The US government retains and the publisher, by accepting the article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Author information
Authors and Affiliations
Contributions
MR: Conceptualization, data curation, writing—editing original draft, Review and editing, visualization, analysis, investigation. BP: Investigation, writing—editing original draft. ML: EPMA characterizations. SD: Conceptualization, writing—editing original draft, investigation, funding.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Romedenne, M., Pint, B., Lance, M. et al. Oxidation Behavior of Heat-Resistant Type HK Steel (HK30Nb) at 800 °C. High Temperature Corrosion of mater. 100, 157–176 (2023). https://doi.org/10.1007/s11085-023-10168-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-023-10168-0