Abstract
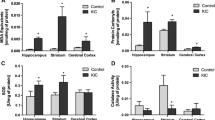
Maple Syrup Urine Disease (MSUD) is a metabolic disease characterized by the accumulation of branched-chain amino acids (BCAA) in different tissues due to a deficit in the branched-chain alpha-ketoacid dehydrogenase complex. The most common symptoms are poor feeding, psychomotor delay, and neurological damage. However, dietary therapy is not effective. Studies have demonstrated that memantine improves neurological damage in neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases. Therefore, we hypothesize that memantine, an NMDA receptor antagonist can ameliorate the effects elicited by BCAA in an MSUD animal model. For this, we organized the rats into four groups: control group (1), MSUD group (2), memantine group (3), and MSUD + memantine group (4). Animals were exposed to the MSUD model by the administration of BCAA (15.8 µL/g) (groups 2 and 4) or saline solution (0.9%) (groups 1 and 3) and treated with water or memantine (5 mg/kg) (groups 3 and 4). Our results showed that BCAA administration induced memory alterations, and changes in the levels of acetylcholine in the cerebral cortex. Furthermore, induction of oxidative damage and alterations in antioxidant enzyme activities along with an increase in pro-inflammatory cytokines were verified in the cerebral cortex. Thus, memantine treatment prevented the alterations in memory, acetylcholinesterase activity, 2′,7′-Dichlorofluorescein oxidation, thiobarbituric acid reactive substances levels, sulfhydryl content, and inflammation. These findings suggest that memantine can improve the pathomechanisms observed in the MSUD model, and may improve oxidative stress, inflammation, and behavior alterations.
Graphical Abstract






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and available upon request.
References
Chuang DT, Shih VE, Max Wynn R (2019) Maple syrup urine disease (branched-Chain Ketoaciduria). In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA (eds) The online metabolic and molecular bases of inherited disease. McGraw-Hill Education, New York, pp 1971–1995
Dancis J, Levitz M, Westall RG (1960) Maple syrup urine disease: branched-chain keto-aciduria. Pediatrics 25:72–79
Strauss KA, Morton DH (2003) Branched-chain ketoacyl dehydrogenase deficiency: maple syrup disease. Curr Treat Options Neurol 5:329–341
Strauss KA, Carson VJ, Soltys K, Young ME, Bowser LE, Puffenberger EG, Brigatti KW, Williams KB, Robinson DL, Hendrickson C, Beiler K, Taylor CM, Haas-Givler B, Chopko S, Hailey J, Muelly ER, Shellmer DA, Radcliff Z, Rodrigues A, Loeven K, Heaps AD, Mazariegos GV, Morton DH (2020) Branched-chain α-ketoacid dehydrogenase deficiency (maple syrup urine disease): treatment, biomarkers, and outcomes. Mol Genet Metab 129:193–206
Sánchez SJ, Visus A, Minana F (2010) Enfermidades de orina de jarabe arce. In: Sanjurjo PBA (ed) Diagnóstico y tratamiento de las enfermedades metabólicas hereditárias 3edn. Eddiciones Ergon, Madrid, p 1332
Strauss KA, Wardley B, Robinson D, Hendrickson C, Rider NL, Puffenberger EG, Shellmer D, Moser AB, Morton DH (2010) Classical maple syrup urine disease and brain development: principles of management and formula design. Mol Genet Metab 99:333–345
Muelly ER, Moore GJ, Bunce SC, Mack J, Bigler DC, Morton DH, Strauss KA (2013) Biochemical correlates of neuropsychiatric Illness in maple syrup urine disease. J Clin Invest 123:1809–1820
Amaral AU, Wajner M (2022) Pathophysiology of maple syrup urine disease: focus on the neurotoxic role of the accumulated branched-chain amino acids and branched-chain α-keto acids. Neurochem Int 157:105360
Taschetto L, Scaini G, Zapelini HG, Ramos ÂC, Strapazzon G, Andrade VM, Réus GZ, Michels M, Dal-Pizzol F, Quevedo J, Schuck PF, Ferreira GC, Streck EL (2017) Acute and long-term effects of intracerebroventricular administration of α-ketoisocaproic acid on oxidative stress parameters and cognitive and noncognitive behaviors. Metab Brain Dis 32:1507–1518
Scaini G, Jeremias GC, Furlanetto CB, Dominguini D, Comim CM, Quevedo J, Schuck PF, Ferreira GC, Streck EL (2014) Behavioral responses in rats submitted to chronic administration of branched-chain amino acids. JIMD Rep 13:159–167
Lemos IS, Wessler LB, Duarte MB, da Silva GL, Bernardo HT, Candiotto G, Torres CA, Petronilho F, Rico EP, Streck EL (2022) Exposure to leucine alters glutamate levels and leads to memory and social impairment in zebrafish. Metab Brain Dis. https://doi.org/10.1007/s11011-022-01070-w
Wessler LB, Farias HR, Ronsani JF, Candiotto G, Dos Santos PCL, de Oliveira J, Rico EP, Streck EL (2019) Acute exposure to leucine modifies behavioral parameters and cholinergic activity in zebrafish. Int J Dev Neurosci 78:222–226
Zinnanti WJ, Lazovic J, Griffin K, Skvorak KJ, Paul HS, Homanics GE, Bewley MC, Cheng KC, Lanoue KF, Flanagan JM (2009) Dual mechanism of brain injury and novel treatment strategy in maple syrup urine disease. Brain 132:903–918
Killian DM, Chikhale PJ (2001) A bioreversible prodrug approach designed to shift mechanism of brain uptake for amino-acid-containing anticancer agents. J Neurochem 76:966–974
Wajner M, Coelho DM, Barschak AG, Araújo PR, Pires RF, Lulhier FL, Vargas CR (2000) Reduction of large neutral amino acid concentrations in plasma and CSF of patients with maple syrup urine disease during crises. J Inherit Metab Dis 23:505–512
Araújo P, Wassermann GF, Tallini K, Furlanetto V, Vargas CR, Wannmacher CM, Dutra-Filho CS, Wyse AT, Wajner M (2001) Reduction of large Neutral amino acid levels in plasma and brain of hyperleucinemic rats. Neurochem Int 38:529–537
Scaini G, Morais MO, Galant LS, Vuolo F, Dall’Igna DM, Pasquali MA, Ramos VM, Gelain DP, Moreira JC, Schuck PF, Ferreira GC, Soriano FG, Dal-Pizzol F, Streck EL (2014) Coadministration of branched-chain amino acids and lipopolysaccharide causes matrix metalloproteinase activation and blood-brain barrier breakdown. Mol Neurobiol 50:358–367
Gold PE (2003) Acetylcholine: cognitive and brain functions. Neurobiol Learn Mem 80:177
Rauca C, Kammerer E, Matthies H (1980) Choline uptake and permanent memory storage. Pharmacol Biochem Behav 13:21–25
Halder N, Lal G (2021) Cholinergic system and its therapeutic importance in inflammation and autoimmunity. Front Immunol 12:660342
Scaini G, de Rochi N, Jeremias IC, Deroza PF, Zugno AI, Pereira TC, Oliveira GM, Kist LW, Bogo MR, Schuck PF, Ferreira GC, Streck EL (2012) Evaluation of acetylcholinesterase in an animal model of maple syrup urine disease. Mol Neurobiol 45:279–286
Sitta A, Ribas GS, Mescka CP, Barschak AG, Wajner M, Vargas CR (2014) Neurological damage in MSUD: the role of oxidative stress. Cell Mol Neurobiol 34:157–165
Sies H (2015) Oxidative stress: a concept in redox biology and medicine. Redox Biol 4:180–183
Teleanu DM, Niculescu AG, Lungu II, Radu CI, Vladâcenco O, Roza E, Costăchescu B, Grumezescu AM, Teleanu RI (2022) An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int J Mol Sci. https://doi.org/10.3390/ijms23115938
Wessler LB, de Miranda Ramos V, Bittencourt Pasquali MA, Fonseca Moreira JC, de Oliveira J, Scaini G, Streck EL (2019) Administration of branched-chain amino acids increases the susceptibility to lipopolysaccharide-induced inflammation in young Wistar rats. Int J Dev Neurosci 78:210–214
Scaini G, Tonon T, de Moura Souza CF, Schuck PF, Ferreira GC, Quevedo J, Neto JS, Amorim T, Camelo JS Jr, Margutti AVB, Hencke Tresbach R, Sperb-Ludwig F, Boy R, de Medeiros PFV, Schwartz IVD, Streck EL (2018) Evaluation of plasma biomarkers of inflammation in patients with maple syrup urine disease. J Inherit Metab Dis. https://doi.org/10.1007/s10545-018-0188-x
Scaini G, Jeremias IC, Morais MO, Borges GD, Munhoz BP, Leffa DD, Andrade VM, Schuck PF, Ferreira GC, Streck EL (2012) DNA damage in an animal model of maple syrup urine disease. Mol Genet Metab 106:169–174
Rosa L, Scaini G, Furlanetto CB, Galant LS, Vuolo F, Dall’Igna DM, Schuck PF, Ferreira GC, Dal-Pizzol F, Streck EL (2016) Administration of branched-chain amino acids alters the balance between pro-inflammatory and anti-inflammatory cytokines. Int J Dev Neurosci 48:24–30
Mescka CP, Wayhs CA, Vanzin CS, Biancini GB, Guerreiro G, Manfredini V, Souza C, Wajner M, Dutra-Filho CS, Vargas CR (2013) Protein and lipid damage in maple syrup urine disease patients: l-carnitine effect. Int J Dev Neurosci 31:21–24
de Medeiros BZ, Wessler LB, Duarte MB, Lemos IS, Candiotto G, Canarim RO, Dos Santos PCL, Torres CA, Scaini G, Rico EP, Generoso JS, Streck EL (2022) Exposure to leucine induces oxidative stress in the brain of zebrafish. Metab Brain Dis 37:1155–1161
Shi X, Ren G, Cui Y, Xu Z (2022) Comparative efficacy and acceptability of cholinesterase inhibitors and memantine based on dosage in patients with vascular cognitive impairment: a network meta-analysis. Curr Alzheimer Res 19:133–145
Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ (2003) Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 348:1333–1341
Bago Rožanković P, Rožanković M, Badžak J, Stojić M, Šušak Sporiš I (2021) Impact of donepezil and memantine on behavioral and psychological symptoms of Alzheimer disease: six-month open-label study. Cogn Behav Neurol 34:288–294
Budni J, Feijó DP, Batista-Silva H, Garcez ML, Mina F, Belletini-Santos T, Krasilchik LR, Luz AP, Schiavo GL, Quevedo J (2017) Lithium and memantine improve spatial memory impairment and neuroinflammation induced by β-amyloid 1–42 oligomers in rats. Neurobiol Learn Mem 141:84–92
Bridi R, Fontella FU, Pulrolnik V, Braun CA, Zorzi GK, Coelho D, Wajner M, Vargas CR, Dutra-Filho CS (2006) A chemically-induced acute model of maple syrup urine disease in rats for neurochemical studies. J Neurosci Methods 155:224–230
Vianna MR, Izquierdo LA, Barros DM, de Souza MM, Rodrigues C, Sant’Anna MK, Medina JH, Izquierdo I (2001) Pharmacological differences between memory consolidation of habituation to an open field and inhibitory avoidance learning. Braz J Med Biol Res 34:233–240
Ellman GL, Courtney KD, Andres V Jr., Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Chao LP, Wolfgram F (1973) Purification and some properties of choline acetyltransferase (EC 2.3.1.6) from bovine brain. J Neurochem 20:1075–1081
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2’,7’-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Bannister JV, Calabrese L (1987) Assays for superoxide dismutase. Methods Biochem Anal 32:279–312
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Tierney AL, Nelson CA (2009) Brain development and the role of experience in the early years. Zero Three 30:9–13
National Research Council, Institute of Medicine Committee on Integrating the Science of Early Childhood (2000). In: Shonkoff JP, Phillips DA (eds) From neurons to neighborhoods: the science of early childhood development. National Academies Press (US), Washington (DC)
Nelson CA, Bloom FE (1997) Child development and neuroscience. Child Dev 68:970–987
Menkes JH, Hurst PL, Craig JM (1954) A new syndrome: Progressive familial infantile cerebral dysfunction associated with an unusual urinary substance. Pediatrics 14:462–467
Cheng A, Han L, Feng Y, Li H, Yao R, Wang D, Jin B (2017) MRI and clinical features of maple syrup urine disease: preliminary results in 10 cases. Diagn Interv Radiol 23:398–402
Klee D, Thimm E, Wittsack HJ, Schubert D, Primke R, Pentang G, Schaper J, Mödder U, Antoch A, Wendel U, Cohnen M (2013) Structural white matter changes in adolescents and young adults with maple syrup urine disease. J Inherit Metab Dis 36:945–953
Neinast M, Murashige D, Arany Z (2019) Branched chain amino acids. Annu Rev Physiol 81:139–164
Sperringer JE, Addington A, Hutson SM (2017) Branched-chain amino acids and brain metabolism. Neurochem Res 42:1697–1709
Smith QR, Momma S, Aoyagi M, Rapoport SI (1987) Kinetics of Neutral amino acid transport across the blood-brain barrier. J Neurochem 49:1651–1658
Gasiorowska A, Wydrych M, Drapich P, Zadrozny M, Steczkowska M, Niewiadomski W, Niewiadomska G (2021) The biology and pathobiology of glutamatergic, cholinergic, and dopaminergic signaling in the aging brain. Front Aging Neurosci 13:654931
Lipton SA, Rosenberg PA (1994) Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 330:613–622
Zhang C, Zhou P, Yuan T (2016) The cholinergic system in the cerebellum: from structure to function. Rev Neurosci 27:769–776
Scaini G, Teodorak BP, Jeremias IC, Morais MO, Mina F, Dominguini D, Pescador B, Comim CM, Schuck PF, Ferreira GC, Quevedo J, Streck EL (2012) Antioxidant administration prevents memory impairment in an animal model of maple syrup urine disease. Behav Brain Res 231:92–96
Calabresi P, Picconi B, Parnetti L, Di Filippo M (2006) A convergent model for cognitive dysfunctions in Parkinson’s disease: the critical dopamine-acetylcholine synaptic balance. Lancet Neurol 5:974–983
Perez-Lloret S, Barrantes FJ (2016) Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. NPJ Parkinsons Dis 2:16001
Winek K, Soreq H, Meisel A (2021) Regulators of cholinergic signaling in disorders of the central nervous system. J Neurochem 158:1425–1438
Islam MT (2017) Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 39:73–82
Forman HJ, Zhang H (2021) Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 20:689–709
Guerreiro G, Mescka CP, Sitta A, Donida B, Marchetti D, Hammerschmidt T, Faverzani J, Coelho Dde M, Wajner M, Dutra-Filho CS, Vargas CR (2015) Urinary biomarkers of oxidative damage in maple syrup urine disease: the L-carnitine role. Int J Dev Neurosci 42:10–14
Mescka CP, Guerreiro G, Hammerschmidt T, Faverzani J, de Moura Coelho D, Mandredini V, Wayhs CA, Wajner M, Dutra-Filho CS, Vargas CR (2015) L-Carnitine supplementation decreases DNA damage in treated MSUD patients. Mutat Res 775:43–47
Hauschild TC, Guerreiro G, Mescka CP, Coelho DM, Steffens L, Moura DJ, Manfredini V, Vargas CR (2019) DNA damage induced by alloisoleucine and other metabolites in maple syrup urine disease and protective effect of l-carnitine. Toxicol in Vitro 57:194–202
Wessler LB, Ise K, Lemos IC, Rezende VL, Duarte MB, Damiani AP, de Oliveira J, de Andrade VM, Streck EL (2020) Melatonin ameliorates oxidative stress and DNA damage of rats subjected to a chemically induced chronic model of maple syrup urine Disease. Metab Brain Dis 35:905–914
Mescka CP, Rosa AP, Schirmbeck G, da Rosa TH, Catarino F, de Souza LO, Guerreiro G, Sitta A, Vargas CR, Dutra-Filho CS (2016) L-Carnitine prevents oxidative stress in the brains of rats subjected to a chemically Induced chronic model of MSUD. Mol Neurobiol 53:6007–6017
Mescka C, Moraes T, Rosa A, Mazzola P, Piccoli B, Jacques C, Dalazen G, Coelho J, Cortes M, Terra M, Regla Vargas C, Dutra-Filho CS (2011) In vivo neuroprotective effect of L-carnitine against oxidative stress in maple syrup urine disease. Metab Brain Dis 26:21–28
Sheldon AL, Robinson MB (2007) The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int 51:333–355
Funchal C, Rosa AM, Wajner M, Wofchuk S, Pureur RP (2004) Reduction of glutamate uptake into cerebral cortex of developing rats by the branched-chain alpha-keto acids accumulating in maple syrup urine disease. Neurochem Res 29:747–753
Pietá Dias C, Martins de Lima MN, Presti-Torres J, Dornelles A, Garcia VA, Siciliani Scalco F, Rewsaat Guimarães M, Constantino L, Budni P, Dal-Pizzol F, Schröder N (2007) Memantine reduces oxidative damage and enhances long-term recognition memory in aged rats. Neuroscience 146:1719–1725
Dąbrowska-Bouta B, Strużyńska L, Sidoryk-Węgrzynowicz M, Sulkowski G (2021) Memantine modulates oxidative stress in the rat brain following experimental autoimmune encephalomyelitis. Int J Mol Sci 22:11330
Sundaram R, Lakshminarayanan G, Rajesh R, Dharmalingam S, Chidambaram K, Ramamurthy S (2013) Neuroprotective potential of Ocimum sanctum (Linn.) leaf extract in monosodium glutamate induced excitotoxicity. Afr J Pharm Pharmacol 7:1894–1906
Emerit J, Edeas M, Bricaire F (2004) Neurodegenerative diseases and oxidative stress. Biomed Pharmacother 58:39–46
Bruunsgaard H, Pedersen M, Pedersen BK (2001) Aging and proinflammatory cytokines. Curr Opin Hematol 8:131–136
Muralidharan S, Mandrekar P (2013) Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol 94:1167–1184
Mescka CP, Guerreiro G, Donida B, Marchetti D, Wayhs CA, Ribas GS, Coitinho AS, Wajner M, Dutra-Filho CS, Vargas CR (2015) Investigation of inflammatory profile in MSUD patients: benefit of L-carnitine supplementation. Metab Brain Dis 30:1167–1174
Sil S, Ghosh T, Ghosh R (2016) NMDA receptor is involved in neuroinflammation in intracerebroventricular colchicine-injected rats. J Immunotoxicol 13:474–489
Mishra A, Kim HJ, Shin AH, Thayer SA (2012) Synapse loss induced by interleukin-1β requires pre- and post-synaptic mechanisms. J Neuroimmune Pharmacol 7:571–578
Amin SN, El-Aidi AA, Ali MM, Attia YM, Rashed LA (2015) Modification of hippocampal markers of synaptic plasticity by memantine in animal models of acute and repeated restraint stress: implications for memory and behavior. Neuromolecular Med 17:121–136
Funchal C, Zamoner A, dos Santos AQ, Loureiro SO, Wajner M, Pessoa-Pureur R (2005) Alpha-ketoisocaproic acid increases phosphorylation of intermediate filament proteins from rat cerebral cortex by mechanisms involving Ca2+ and cAMP. Neurochem Res 30:1139–1146
Funchal C, de Lima Pelaez P, Loureiro SO, Vivian L, Dall Bello Pessutto F, de Almeida LMV, Tchernin Wofchuk S, Wajner M, Pessoa Pureur R (2002) α-Ketoisocaproic acid regulates phosphorylation of intermediate filaments in postnatal rat cortical slices through ionotropic glutamatergic receptors. Dev Brain Res 139:267–276
Wang C, Jensen FE (1996) Age dependence of NMDA receptor involvement in epileptiform activity in rat hippocampal slices. Epilepsy Res 23:105–113
Funding
This research was supported by the Higher Education Personnel (CAPES), the Brazilian National Council for Scientific and Technological Development (CNPq), the Santa Catarina State Research and Innovation Support Foundation (Fapesc), and the University of Southern Santa Catarina (UNESC).
Author information
Authors and Affiliations
Contributions
Conception and design of the research: ISL, GL, ELS. Acquisition of data: ISL, HTB, EP, CAT, CGA, RTM, RFS, APP. Analysis and interpretation of the data: ISL, RAMÁ, GZR, EPR, ELS. Obtaining financing: ELS. Writing of the manuscript: ISL. Critical revision of the manuscript for intellectual content: GL, ELS.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Ethical Approval
The experimental procedures were previously approved by the ethics committee of the UNESC (Protocol number 30/2020 and 37/2021) and followed the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lemos, I.S., Torres, C.A., Alano, C.G. et al. Memantine Improves Memory and Neurochemical Damage in a Model of Maple Syrup Urine Disease. Neurochem Res 49, 758–770 (2024). https://doi.org/10.1007/s11064-023-04072-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-04072-x