Abstract
Temporal lobe epilepsy is the most drug-resistant type with the highest incidence among the other focal epilepsies. Metabolic manipulations are of great interest among others, glycolysis inhibitors like 2-deoxy d-glucose (2-DG) being the most promising intervention. Here, we sought to investigate the effects of 2-DG treatment on cellular and circuit level electrophysiological properties using patch-clamp and local field potentials recordings and behavioral alterations such as depression and anxiety behaviors, and changes in nitric oxide signaling in the intrahippocampal kainic acid model. We found that epileptic animals were less anxious, more depressed, with more locomotion activity. Interestingly, by masking the effect of increased locomotor activity on the parameters of the zero-maze test, no altered anxiety behavior was noted in epileptic animals. However, 2-DG could partially reverse the behavioral changes induced by kainic acid. The findings also showed that 2-DG treatment partially suppresses cellular level alterations while failing to reverse circuit-level changes resulting from kainic acid injection. Analysis of NADPH-diaphorase positive neurons in the CA1 area of the hippocampus revealed that the number of positive neurons was significantly reduced in dorsal CA1 of the epileptic animals and 2-DG treatment did not affect the diminishing effect of kainic acid on NADPH-d+ neurons in the CA1 area. In the control group receiving 2-DG, however, an augmented NADPH-d+ cell number was noted. These data suggest that 2-DG cannot suppress epileptiform activity at the circuit-level in this model of epilepsy and therefore, may fail to control the seizures in temporal lobe epilepsy cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is one of the most complicated neurological diseases that is characterized by neuronal hyperexcitability and sudden, simultaneous discharges which appear as seizures. Almost 1% of the general population are diagnosed with epilepsy, and about 40% of the cases are pharmacoresistant. Temporal lobe epilepsy has the highest incidence among the other types of epilepsy and exhibits considerable resistance to antiseizure agents [1]. Epilepsy is associated with many behavioral disorders, including anxiety and depression, as reported in epileptic patients and animal models; therefore, introducing novel and potent approaches and compounds to control seizures and behavioral comorbidities is of paramount importance.
Among many novel strategies, metabolic manipulations have widely attracted attention as the ketogenic diet (KD), low carbohydrate and high-fat diet is shown to have been effective in many drugs resistant cases. KD acts through a great many mechanisms among which, the most important is to cut out glycolysis and subsequent attenuation of lactate shuttle between neurons and astrocytes [1] and, more importantly, decreased cytosolic ATP concentration. Lactate dehydrogenase inhibition which results in direct inhibition of glycolysis is demonstrated to have suppressed interictal discharges in the intrahippocampal kainic acid model of temporal lobe epilepsy, which is one of the most drug-resistant models [2].
2-deoxy d-glucose (2-DG) is another glycolysis inhibitor that has been studied over the last few years for its possible therapeutic actions [3,4,5,6,7]. However, interestingly, both its proconvulsant and anticonvulsant effects have been observed according to the different methods of epilepsy induction in animals. For instance, in i.v PTZ, i.v kainic acid and electroshock-induced seizures, 2-DG decreased seizure threshold while in the 6-Hz seizure test it led to seizure threshold increment [5]. Furthermore, in pilocarpine-induced epilepsy, while elevating seizure latency, 2-DG diminished seizure duration and severity [8]. In in-vitro models like high [K]o, likewise, 2-DG dwindled interictal epileptiform activity [9]. 2-DG is of interest not only for its possible anticonvulsive implication but also for its potency in the inhibition of cancerous cell growth. Indeed, it is already in clinical use for treating SARS-Cov-2 [10] and has had promising results in the suppression of cancerous cell growth in clinical trials [11]. Therefore, owing to the major inconsistency between the effects of 2-DG in different epilepsy models, and more importantly, given that models like i.v kainic acid could not be considered as focal temporal lobe epilepsy [12], more preclinical studies are required to report the extend of 2-DG’s potency in suppressing the seizures and behavioral comorbidities in a model that may potentially resemble human temporal lobe epilepsy.
The intrahippocampal kainic acid model of temporal lobe epilepsy, which is deemed as an appropriate simulator of human temporal lobe epilepsy due to hippocampal sclerosis seen in this model [13], has long been used to assess the therapeutic effects of nominated compounds to control the seizures and/or treat epilepsy. Although a previous study has reported that comorbid psychiatric symptoms including, anxiety and depression behaviors are not significantly different between control and epileptic animals in this model [14], severe cell loss in the dorsal hippocampus and general ipsilateral hippocampal deformation plants doubt in the mind whether follow-up tests would probably bring out different results. Previous lesion studies have posited a link between dorsal and ventral hippocampus lesions, which are seen in this model, and diminished anxiety levels [15]. Additionally, shriveled hippocampus is associated with depression behavior [16].
Considering previous observations, the present study aimed to further explore the potency of 2-DG in reversing the electrophysiological (at the circuit and cellular levels), behavioral, and histochemical consequences of intrahippocampal kainic acid injection in mice which bears a striking resemblance to human temporal lobe epilepsy; we hypothesized that 2-DG can suppress behavioral (if present), electrophysiological and histochemical alterations following temporal epilepsy induction.
To test this hypothesis, Local Field Potentials (LFP) and patch-clamp recordings were used to assess the circuit and cellular level effects of 2-DG respectively. Interictal epileptiform activity is a known characteristic of intrahippocampal kainic acid model of temporal lobe epilepsy and suppression of such activity has been widely used to assess the efficiency of different antiseizure candidates [17]. Burst activity, as well as hyperexcitability of survived neurons in intrahippocampal kainic acid model of temporal lobe epilepsy, is noted [18, 19]; we evaluated passive membrane properties and spontaneous activity of the survived dorsal CA1 pyramidal neurons and how 2-DG affected intrahippocampal kainic acid model of temporal lobe epilepsy induced alterations. Moreover, we addressed how behavioral comorbidities like anxiety and depression, if present, were affected by 2-DG injection. Zero-maze test was used to evaluate the anxiety status of the animals; an open field test was performed to assess locomotion status as well as fear/anxiety behavior. Sucrose preference test which is an indicator of anhedonia, the core symptom of depression [20, 21], was done to evaluate depression in the animals. Furthermore, since the nitrergic system is involved in the regulation of excitatory and inhibitory neurotransmission, and becomes imbalanced during epileptogenesis [22, 23], we also evaluate alterations in the NOergic neurotransmission by using NADPH-diaphorase staining.
As Duveau and colleagues reported in 2016 [17], most of the neuropathological and electrophysiological features that are seen in human MTLE can be reproduced in the kainic acid mouse model of TLE. Therefore, this animal model of TLE is deemed to be a potent simulator of human temporal lobe epilepsy owing to severe cell loss and sclerosis noted in the hippocampus and, more importantly, chronic spontaneous seizures following kainic acid injection. Here, unilateral injection of kainite into mouse hippocampus was used to evaluate the beneficial effects of 2-DG against TLE, which is already in clinical use for suppression of cancerous cell growth and covid-19 treatment. 2-DG has also been in various stages of preclinical and clinical development for epilepsy for several years. The present work tried to extend the preclinical profile of 2-DG to better understand how this agent can exert beneficial effects. If potent, hence, inhibition of glycolysis by 2-DG could be considered as a therapeutic intervention for the treatment of epileptic patients with drug resistance.
Materials & Methods
Animals
This study was carried out on 64 adult male NMRI mice (weighing 30–35 g; Pasteur institute, Tehran). The animals were housed with free access to standard pellet diet and tap drinking water ad libitum. They were kept in a temperature-controlled (23 ± 2 °C) animal house free from any source of chemical or noise pollution under the 12:12 h light: dark cycle. All animals received human care and gentle handling throughout the study, as it has been shown that proper techniques and frequency of handling were used to reduce stress and anxiety [24, 25]. Mice were single housed after the surgery; although social housing is deemed to be the optimal way of housing, previous studies showed that single housing does not significantly affect behavioral tests in mice [26]. Hence, we single-housed the mice in order not to arouse aggression, especially in epileptic animals. All experimental procedures and animal care were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Biomedical Research Ethics Committee of the National Institute for Medical Research Development (Approval ID: IR.NIMAD.REC.1399.259) and the Ethics Committee of Shahid Beheshti University of Medical Sciences (Authorization Code: IR.SBMU.MSP.REC.1400.630).
Study Design
The present study intended to investigate the behavioral, electrophysiological and histochemical consequences of glycolysis inhibition on the intrahippocampal kainic acid model of temporal lobe epilepsy. Three separate groups of experiments were conducted to assess the effects of glycolysis inhibition by 2-DG on: (1) Kainic acid-induced hyperexcitability in CA1 pyramidal neurons using patch-clamp recording, (2) Local field potential (LFP) recordings to measure epileptiform activity, and (3) Behavioral tests to assess the locomotor activity, anxiety and depression behaviors. To assess histological alterations, however, the animals were randomly chosen from the animals which had undergone behavioral tests.
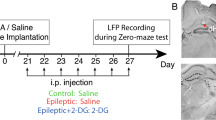
Kainic acid was stereotaxically microinjected into the dorsal hippocampus of the left hemisphere (day 0, for more details, see the epilepsy induction section) and 2-DG (300 mg/kg, i.p) was injected 3 weeks after IHKA injection for 7 days (Fig. 1) and the last injection was given 90 min before starting the experiments. This time was chosen based on the study done by Koenig et al., 2019, who reported that 90 min after 2-DG injection, induction of ketosis is observed [27].
The dose of 2-DG was chosen based on previous studies that report the anticonvulsant and antiepileptic action of 2-DG [5, 9].
The animals randomly were divided into the following five groups: Control group, a group in which the mice underwent stereotaxic surgery and received intrahippocampal saline (40 nL) injection ( as a solvent for kainic acid) on the 0th day; Control + 2-DG group, a group that mice received intrahippocampal saline (40 nL) on the 0th day and i.p injection of 300 mg/kg 2-DG from the 20th to 28th day, once a day; Epileptic group, a group in which the mice received an intrahippocampal injection of kainic acid on the 0th day; Epileptic + 2-DG group: a group in which epileptic mice treated i.p with 2-DG at the dose of 300 mg/kg from the 20th to 28th day, once a day; epileptic + saline group: a group that epileptic mice received 300 mg/kg i.p saline from the 21st to 27th day, once a day.
The order of the behavioral tests was as follows: zero maze, open field and sucrose preference test on the 20th, 21st, and 22nd days respectively in control and epileptic groups; in epileptic + 2-DG and control + 2-DG groups, however, the tests were performed on the 26th, 27th, and 28th days respectively (Fig. 1A). Since in the behavioral study, we did not find a significant difference between control groups and control + 2-DG groups (for more details see results), and according to the animal ethics guidelines to minimize the number of animals used, this group was omitted in LFP and patch-clamp recordings. Due to the fact that saline and handling did not lead to a significant alteration in LFP groups, we omitted epileptic + saline groups from patch-clamp recordings (Fig. 1C). It should be noted that according to previous studies in our laboratory, saline and surgery do not lead to considerable variation between the groups; hence, the study does not contain intact and sham groups [28].
Epilepsy Induction
Temporal lobe epilepsy was induced as previously described by Sada et al. [2]. Briefly, mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) and fixed in the stereotaxic frame. Then, 0.8 nmol kainic acid was dissolved in 40 nL normal saline and directly injected into the left dorsal hippocampus (− 1.6 mm to the Bregma, 1.6 from the midline, and 1.2 mm deep from the dura mater) according to the atlas of Paxinos and Franklin (2001). Due to the non-convulsive status epilepticus, verification of model induction was endorsed by frequent interictal epileptiform activity (sharp-wave complexes) (see below for details) as well as severe cell loss in the dorsal CA1 pyramidal cell layer (see Nissl Staining) (Fig. 2C, D). After the experiments, the anesthetized animals were decapitated and the brains were dissected out for injection site verification (Fig. 2A).
Verification of injection site, electrode site, and cell loss following kainic acid injection. A the injection site of kainic acid or saline. B electrode location in LFP group animals. C normal dorsal hippocampus (control); D severe cell loss in CA1 and, to less extent, in CA3 as well as swollen dentate gyrus in kainic acid-treated hippocampus compared to the control hippocampus. Scale bar: 300 µm
Behavioral Tests
Open Field Test
To measure the locomotor activity, animals were placed in the center of an open field (35 × 35 × 35 cm) after half an hour of habituation to the experimental room. In the following ten minutes, moved distance and velocity were analyzed by EthoVision XT 11 software. To assess thigmotaxis, which is the tendency of an animal to stay near the walls of the open field, a center (15 × 15 × 15) was defined and time spent in the center was analyzed. The arena was cleaned with ethanol 85% between the trials.
Zero-Maze Test
Anxiety behavior was evaluated by using zero-maze apparatus [29]. The apparatus (60 cm in diameter, 5 cm wide circular corridor, 16 cm high walls and 60 cm high from the floor) was made of wood and painted dark black. After half an hour of habituation to the experiment room, each animal was placed in an open arm-closed arm intersection, facing the closed arm. During the following 5 min, the animal was videotaped and analyzed offline afterwards. Five parameters were assessed, as described before by Shepherd [30] including the time spent in the open arms, the number of entries to the open arm, the latency of the first open arm entry, head dipping frequency, and body stretching frequency. Between the trials, the apparatus was cleaned using 70% ethanol.
Sucrose Preference Test
Rodents are shown to prefer sweet water rather than tap water. Suffering from depression, however, they tend to consume less sweet water in comparison with tap water in normal conditions [21]. To perform the test, the animals were given access to two tap water bottles for 24 h as habituation in their home cage. During the next 24 h, both bottles were taken and replaced with two new bottles, one containing 3% sucrose solution while the other filled with tap water. As the diameter of the drinking hole had been noted to influence on the amount of water consumed by the animal [31], the holes were all equalized in size using a 2 mm drill. The proportion of sweet/tap water consumption was calculated afterwards.
Local Field Potentials (LFP) Recordings
Mice underwent stereotaxic surgery to record local field potentials. They were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). The ear bars were placed delicately prior to muzzle fixation. Lidocaine 2% was injected under the scalp skin 5 min before making an approximately 2 cm incision in the skin. Following Bregma-Lambda adjustment to a plane level, three holes were made by a fine drill. To prepare electrodes, two stainless steel wires (127 μm in diameter, A.M. system Inc., USA) were intertwined to give the electrode suitable strength and flexibility. The electrode, then, was soldered to a connector and placed in the dorsal hippocampus (− 2.1 mm AP, 1.5 mm ML, 1.2 mm DV). Six screws (one as the reference electrode above the cerebellum) were screwed to the scalp. Lastly, dental cement was used to fix the electrodes. The LFP signals were continuously recorded for 13 h at 1 kHz sample rate and low-pass filtered at 250 Hz while the animals were freely moving. Interictal epileptiform discharges were defined as sharp-waves, having more than twofold amplitude compared with baseline, as well as having a frequency between 1 and 20 Hz. The discharges were detected and analyzed by MATLAB 2016 software. At the end of the experiments, brains were removed to verify the proper placement of the electrode (Fig. 2B).
Patch-Clamp Recording
To investigate the possible cellular-level effects of epilepsy induction by kainic acid on the electrophysiological properties of hippocampal CA1 pyramidal neurons, and whether 2-DG can reverse these possible alterations, whole-cell patch-clamp recording was performed as follows. Briefly, the animals were deeply anaesthetized with ether and then decapitated. The brains were removed immediately and placed in ice-cold artificial CSF (ACSF) containing (in mM): 206 sucrose, 2.8 KCl, 1 CaCl2, 1 MgCl2, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 10 d-glucose, and saturated with 95% O2 and 5% CO2 (pH 7.3–7.4; 300 mOsm). Transverse slices (300 μm) were cut by using a vibroslicer (7000 smz-2, Campden Instruments Ltd, UK). Slices were placed in a holding chamber containing ACSF composed of (in mM) 125 NaCl, 2.5 KCl, 1.5 CaCl2, 1.25 NaH2PO4, 25NaHCO3, 10 d-glucose, pH 7.4, 300 mOsm for at least 60 min at 32–35 °C. The slices were kept at room temperature (23–25 °C) before transferring to the recording chamber. After incubation for at least 1 h, each slice was individually transferred to a submerged recording chamber on the stage of an upright microscope (BX51WI, Olympus); they were continuously superfused with oxygenated ACSF at a rate of 2–3 ml/min at 23–25 °C afterwards. Patch pipettes [borosilicate glass capillary (1.5 mm O.D., 0.86 mm I.D)] were pulled with a PC10 two-stage vertical puller (Narishige, Japan). The pipettes’ resistance was 3–6 MΩ when filled with an internal solution containing (in mM): 135 potassium gluconate, 10 KCl, 10 HEPES, 1 MgCl2, 2 Na2ATP and 0.4 Na2GTP. The pH of the internal solution was set to 7.3 by KOH, and the osmolarity was adjusted to 290 mOsm. Whole-cell patch-clamp recordings were performed using Multiclamp 700B amplifier equipped with Digidata 1322A data acquisition board, and pClamp nine software (Axon, Molecular Devices, CA, USA). All recordings were done from CA1 pyramidal neurons in current-clamp mode. The recordings were filtered at 5 kHz, sampled at 10 kHz and stored on a personal computer for offline analysis. The data were analyzed offline by using clampfit version 11.2 (Molecular devices) and MATLAB 2016 software.
The passive electrical properties of the CA1 pyramidal neurons were measured by applying hyperpolarizing current pulses (− 50 to − 400 pA, 800 ms). The resting membrane potential was recorded after the initial break-in of the cell membrane. To obtain input resistance, the current–voltage curve was drawn and its slope was measured as the resistance using the first four sweeps (0 to − 150 pA). The membrane time constant (tau) was evaluated by the exponential fitting of capacitive voltage relaxation. Further, membrane capacitance was obtained by dividing the time constant by the input resistance.
Spontaneous activity was recorded and analyzed in a 60 s epoch. The firing regularity was quantified by the coefficient of variation (CV) of the ISI (inter-spike interval) which was calculated as the ratio of the standard deviation to the mean of ISI. The amplitude of AHP was measured from the threshold to the peak of the hyperpolarization following the action potential. To investigate the impact of kainic acid and 2-DG injection on rebound APs, a hyperpolarizing ramp current (1000 ms, − 300 pA with a slope of 0.345 pA/ms) followed by a depolarizing current pulse (100 pA for 300 ms) was applied. Burst activity was assessed in 125 s epochs.
Histochemical Assessment
Nissl Staining
To show the brain injury induced by intrahippocampal injection of kainic acid, Nissl staining was performed. Following anesthesia (100 mg/kg ketamine and 10 mg/kg xylazine), transcardial perfusion was performed with saline and 4% paraformaldehyde, 1.33% picric acid in 0.1 M phosphate buffer (pH 7.4). Then, the mice were decapitated and brains were removed and post-fixed in the same fixative. To verify injection and electrode sites, the brains were cryoprotected in 20% sucrose buffer at 4 °C overnight. Coronal Sects. (20 μm) containing the hippocampus were serially cut using a cryostat (Leica CM1850, Germany). However, to evaluate cell loss, the brain blocks were processed and embedded in paraffin and 8 µm sections were obtained using rotary microtome apparatus (Cut5062, Germany) and mounted on gelatin-coated slides. Nissl staining (0.1% Cresyl violet) was performed afterwards. To assess morphological properties of the CA1 pyramidal neurons (diameter of the soma), the long axis length of the soma was measured in the neurons containing a visible nucleus, nucleolus, and primary dendritic cone (from the neck of the dendritic cone to the opposite pole of the soma) using a computer-based image analysis system (Olympus BX60, DP12, Olysia Soft Imaging System, Japan).
NADPH Diaphorase Staining
The mice were anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine, i.p) on the day 21st (epileptic and control groups) or 29th (epileptic + 2-DG group) (Fig. 1A) and were perfused transcardially with a cold fixative containing 4% paraformaldehyde and 1.33% picric acid in 0.1 M phosphate buffer (PB, pH 7.4) following 0.9% saline perfusion. The brains were then dissected out from the skull, post-fixed overnight in the same fixative at 4 °C and cryoprotected by being immersed in 20% sucrose until they sank. The brains were freeze-sectioned coronally at 50 µm thickness, between the AP 1.2 and 2.4 mm posterior to the Bregma (Paxinos and Franklin, 2001) using a cryostat (Leica CM1850, Germany). NADPH-d staining was performed by incubating free-floating sections in the light-protected 0.1 M PB (pH 7.4) solution, containing 1 mg/ml nicotinamide adenine dinucleotide phosphate diaphorase (β-NADPH-d), 0.1 mg/ml nitroblue tetrazolium (NBT), and 0.3% Triton X-100 (all reagents were obtained from Sigma, St. Louis, MO, USA) at 37 °C for 1–2 h. The sections, then, were mounted on the gelatin-coated slides and cover-slipped with Entellan. Seven sections from the anterior–posterior axis of hippocampal CA1 area per animal were examined under light microscopy to localize NADPH-d+ neurons. The NADPH reactive cells were photomicrographed by the same Olympus microscope as mentioned above and manually counted.
Statistical Analysis
SPSS 26 (IBM SPSS Statistics. Armonk, NY: IBM Corp) and GraphPad Prism 8 software (GraphPad, La Jolla, CA, USA, respectively) were employed to compare the data between groups and significance levels. One-way ANOVA and student’s t-test were used to make a comparison between independent variables while the ANCOVA test was utilized to mask the effect of locomotion on anxiety behavior (see results and discussion). Pearson’s test (or Spearman’s test when a non-parametric test was needed) was employed to assess the correlation between the variables. Numerical data were expressed as mean ± standard error of the mean (SEM) and a value of P < 0.05 was considered statistically significant.
Results
Behavioral Tests
Epileptic Animals Showed Increased Locomotor Activity, but 2-DG Treatment was not able to Reverse Kainic Acid-Induced Enhancement in Locomotor Activity in the Epileptic Group
The epileptic and epileptic + 2DG animals travelled a significant longer distance (N = 8 in each group; P < 0.05 for both) than the control group (N = 8; Fig. 3A). Note, that there was no significant difference between control and control + 2-DG groups. Moreover, thigmotaxis was not significantly different between the four groups indicating no substantial change in fear-motivated and anxiety behavior of the epileptic animals (Fig. 3A, C). Furthermore, the average velocity of the movement during exploration in the open field was significantly higher in both epileptic and epileptic + 2DG than in control (P < 0.05 for both; Fig. 3B) indicating more locomotor activity in epileptic and epileptic + 2-DG animals, and 2-DG treatment could not reverse the changes.
Decreased anxiety behavior as well as increased depression behavior in epileptic animals. A heat maps presenting thigmotaxis in the groups, B indicates increased travelled distance in epileptic (N = 8) and epileptic + 2-DG (N = 8) groups respectively compared to the control (N = 8) animals. C spent time in the center zone, which was not significantly different between the groups. D increased velocity of locomotion in epileptic and epileptic + 2-DG groups. Note that 2-DG was unable to reverse altered locomotion induced by kainic acid injection. E increased spent time in the open arms in epileptic and epileptic + 2-DG groups compared to control group. F A significant increase in the number of open arm entries in epileptic animals; 2-DG partially reversed the alterations. G latency of first open arm entry was substantially lower in epileptic animals compared to the control group. The epileptic + 2-DG group showed lower latency as well but did not reach the significance level. H head dipping frequency was higher in epileptic and epileptic + 2-DG animals in comparison with control groups. I body stretching frequency was not significantly different between the groups. J Sucrose preference is shown to be diminished in epileptic animals, 2-DG partially reversing it. Notably, 2-DG led to a slight alteration in the test results in control + 2-DG (N = 6) group compared to the control animals. The bars represent the mean ± SEM. *P < 0.05, **P < 0.01
Epileptic Animals Expressed Reduced Anxiety Behavior, 2-DG Only Slightly Reversing the Changes
Less anxiety-like behavior in the epileptic animals compared to control group was extrapolated from more time spent in the open arms (P < 0.01; Fig. 3C), more open arm entry (P < 0.01; Fig. 3D), smaller latency of first entry to open arm (P < 0.01; Fig. 3E), and more head dipping frequency (P < 0.05; Fig. 3F). Nevertheless, body stretching frequency, which is deemed to reflect anxiety level the most [30] was not significantly different between the groups (Fig. 3G). Even though 2-DG seems to have slightly reversed the alterations (number of open arm entry and latency of first open arm entry), the drug group cannot resemble the control group. 2-DG had a similar effect on control + 2DG animals as it had on the epileptic animals (Fig. 3).
Increased Locomotor Activity Seems to be the Main Player in the Zero-Maze Test
ANCOVA (analysis of covariance) revealed that the locomotion state of the animals affects the zero-maze parameters as a covariate. Pearson’s test (or Spearman’s test) demonstrated a significant correlation between moved distance in the open field and all the zero-maze parameters except for body stretching frequency; there was no interaction between the group and locomotor variables, hence, a necessary prerequisite of ANCOVA test was met. Masking locomotion’s effect by the ANCOVA test, animals in the control, epileptic, and epileptic + 2-DG showed no significant difference in the parameters of anxiety behavior but head dipping frequency (Table1).
Epileptic Animals Appeared to Present with Depression-like Behavior, 2-DG Partially Leading to more Sweet Water Consumption
The ratio of sweet water to tap water consumption was lower in the epileptic animals, being partially reversed in the epileptic group receiving 2-DG (P < 0.05; Fig. 3H). It is worth noting that depression-like behavior deduced from the result of this test suggests an all-or-none pattern. It is to say, each epileptic animal was either depressed (tap water consumption was much higher than sweet water consumption) or not depressed (sweet water consumption was much higher than tap water consumption). 2-DG had no considerable effect in the control + 2-DG group (Fig. 3H).
Electrophysiology
2-DG Failed to Suppress Interictal Sharp-Wave Complexes
A preferential enhancement of glycolysis in the activated brain during diseases, like epilepsy, has been reported [4] and based on this, the antiseizure effect of glycolysis inhibition has been proposed [5, 7, 9, 23]. Therefore, we next examined the electrophysiological consequences of 2-DG treatment, which inhibits glycolysis competitively and prevents ATP production, in a kainic acid model of temporal lobe epilepsy.
13 h of continuous LFP recording was conducted from the dorsal hippocampus of the epileptic animals. Sharp-waves were frequently seen in the epileptic group while were never observed in control animals (Fig. 4A, B). Analysis of these sharp-wave complexes revealed no significant difference in the ratio of total sharp-wave complex time (Fig. 4C), the ratio of mean sharp wave duration (Fig. 4D), and sharp wave frequency (Fig. 4E) between the epileptic + 2-DG (N = 5) and epileptic + saline (N = 5) groups. The ratios were obtained by division of the quantities taken on the 21st and 28th days in each group (28th/21st). Although the parameters are reported for the whole 13 h, the sharp-wave complexes were scrutinized in one-hour epochs during the whole 13 h and no notable suppression was noted (data not shown). LFP results suggest that 2-DG is not able to suppress epileptiform activity at the circuit-level.
Interictal epileptiform activity and 2-DG’s circuit-level effect. A and B 10-min epochs from control and epileptic animals respectively, indicating interictal epileptiform discharges (sharp-wave complexes) (five complexes are seen in this epoch). These complexes were never seen in control animals. The ratios of total sharp-wave complex time C, sharp wave frequency (frequency of the discharges within a complex) D, and sharp-wave duration (mean duration of each complex) E were not significantly different between epileptic + 2-DG and epileptic + saline groups during 13 h of recording. Epileptic animals (N = 5) were recorded on the day 21th (3 weeks after epilepsy induction), then received 2-DG for a week and recorded on the day 28th again (epileptic + 2-DG group). epileptic + saline group (N = 5) was added to figure out whether the increment in epileptiform activity following 2-DG injection was a result of 2-DG or progression of the pathology. The ratios were obtained by dividing the data acquired on day 28th by the data on day 21th. Data are shown as mean ± SEM
Glycolysis Inhibition by 2-DG Cannot Completely Reverse Epilepsy-Induced Cellular Electrophysiological Changes in the CA1 Pyramidal Neurons
By applying hyperpolarizing currents (Fig. 5A) patch-clamp findings showed that resting membrane potential remained unchanged either following induction of epilepsy or inhibition of glycolysis in the epileptic group (N = 6) and epileptic + 2-DG group (N = 7), when compared to control group (N = 8; Fig. 5B). The membrane resistance was also not affected by intrahippocampal kainic acid injection and 2-DG treatment (Fig. 5C). However, the time constant was shorter in both epileptic and epileptic + 2-DG groups (P < 0.01 and P < 0.001, respectively; Fig. 5D) compared to control group. Furthermore, the membrane capacitance was significantly decreased in these groups (P < 0.01 and P < 0.05, respectively; Fig. 5E) when compared to the control neurons. Changes in membrane capacitance were associated with a significantly smaller cell size of survived pyramidal neurons (Figs. 5F–H) both in the epileptic (N = 4, P < 0.05) and in the epileptic plus 2-DG group (N = 4, P < 0.05; Fig. 5I). As it can be seen in Nissl-stained sections, induction of epilepsy also led to a remarkable cell loss in the epileptic group and inhibition of glycolysis did not stop the loss of neurons in the epileptic group receiving 2-DG (Fig. 2D).
The electrophysiological consequences of Kainic acid and 2-DG treatment on the Passive properties of dorsal CA1 pyramidal cell membranes. A hyperpolarizing currents to measure membrane resistance, capacitance and tau in control (upper) (N = 8), epileptic (middle) (N = 6) and epileptic + 2DG (N = 7) groups. resting membrane potential B as well as membrane resistance C, were similar between the three groups. Membrane tau D and capacitance E, however, were significantly lower in epileptic and epileptic + 2-DG groups compared to control animals. The pyramidal layers of CA1 are shown in control F, Epileptic G, and epileptic + 2-DG H groups. Note that only a small proportion of CA1 pyramidal neurons have survived following kainic acid injection (also see Fig. 1). I Demonstrates the diminished size of the survived cells in epileptic and epileptic + 2-DG groups. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bar: 50 µm
Whole-cell current-clamp recordings revealed that neurons from epileptic mice (N = 3) showed enhanced neuronal excitability when compared to control cells (Fig. 6A) and exhibited a significant higher firing frequency (P < 0.05; Fig. 6C) but treatment with 2-DG(N = 5) following induction of epilepsy reduced the neuronal excitability and firing frequency (Figs. 6A, C).
Spontaneous activity and action potential properties of dorsal CA1 pyramidal neurons. A 15 s epochs from the spontaneous activity of control (upper) (N = 5), epileptic (middle) (N = 3), and epileptic + 2-DG (N = 5) groups. Note the high frequency of action potential firing in the epileptic group, being highly reversed by 2-DG. B superimposed APs from the three groups clearly indicating the alterations (see below). Note the afterdepolarization (ADP) only in epileptic + 2-DG group (b). Dramatically increased frequency of AP firing C in a 60 s epoch in epileptic animals compared to the control group; 2-DG, however, reversed the changes substantially. D AP amplitude augmented significantly compared to both epileptic and control groups. AP rising tau was significantly lower in epileptic + 2-DG group compared to control animals. Although not reaching to significance level, it was lower in epileptic group compared to epileptic + 2-DG group as well E. Even though AP half-width was much similar in the three groups F, AP duration was significantly higher in 2-DG treated epileptic animals G owing to ADP noted only in the epileptic + 2-DG group. H Afterhyperpolariziation, although being lower in the epileptic and epileptic + 2-DG groups, did not reach to significance level between the three groups. I 2-DG led to increased irregularity compared to the epileptic and control groups. J latency of rebound APs is significantly smaller in epileptic and epileptic + 2-DG groups compared to control animals. K Superimposed rebound APs following a hyperpolarizing ramp current. APs occurred after injection of + 100 pA current following the ramp current (dashed line); the APs are shown with a different time scale to show the latency of APs following the injections. The bars represent the mean ± SEM. *P < 0.05, **P < 0.01
Furthermore, although the amplitude of action potential was not affected by epilepsy induction when compared with the control group, inhibition of glycolysis in the epileptic mice that received 2-DG resulted in a significant increase in the AP amplitude (N = 4, P = 0.033; Fig. 6D, B). Induction of epilepsy following intrahippocampal injection of kainic acid led to a slightly slower depolarization phase of the action potential, as evidenced a non-significant increase in the rising tau compared to control cells, but 2-DG treatment resulted in a significantly faster time constant of the rising phase of the action potential, as compared both to the control and epileptic groups (P < 0.05; Fig. 6E). We also found that either induction of epilepsy or 2-DG treatment had no significant effect on the AP half-width (Fig. 6F). Nevertheless, due to afterdepolarization (ADP), which is only noted in the epileptic + 2-DG group (Fig. 5b), the duration of AP (measured at threshold voltage) was significantly increased both in epileptic and epileptic + 2-DG groups compared to control group (P < 0.05; Fig. 6G). The amplitude of AHP did not differ significantly between the groups (Fig. 6H). Inhibition of glycolysis, but not induction of epilepsy alone, was accompanied by a significant increase in the coefficient of variation of interspike interval (ISI) when compared to either control (P < 0.05) or epileptic (P < 0.05; Fig. 6I) groups. This may indicate the irregularity of the firing pattern of the CA1 pyramidal neurons in epileptic mice that received 2-DG.
The latency of onset of the first post-inhibitory rebound spike, following a hyperpolarizing ramp current, was significantly shorter in the epileptic neurons than in the control ones (P < 0.01); likewise, it was significantly lower in the epileptic + 2-DG group compared to the control group (P < 0.05, Fig. 6J, K).
To further assess the impact of 2-DG treatment on the firing pattern, we analyzed quantitative burst activity. Burst activity in hippocampal pyramidal neurons has already been demonstrated in epileptic cells [32]. Here, neurons obtained from all the epileptic animals showed severe burst activity (Fig. 7A); in the epileptic + 2-DG group, however, neurons from 2 of the 5 animals showed no burst activity; the other three animals showed attenuated burst activity compared with the epileptic animals (Fig. 7B). Even though the difference between the parameters did not reach to the significance level, the number of bursts decreased by 61% (Fig. 7C), the mean duration of each burst diminished by 207% (Fig. 7D), the mean AP number in each burst decreased by 71% (Fig. 7E), and the mean pause between the bursts dwindled by 41% (Fig. 7F). Patch-clamp results, hence, suggest that 2-DG could notably reverse the alterations that ensued from epilepsy induction at the cellular level. Moreover, it led to alterations in AP properties that did not assemble the control animals.
Burst activity suppression by 2-DG. A and B 15 s epochs from epileptic and epileptic + 2-DG animals. In 2 out of 5 epileptic + 2-DG animals burst activity was never seen. Mean burst number, mean AP number in each burst, mean burst time, and mean pause between two bursts did not reach to the significance level between the two groups in 125-second epochs C–F. Nevertheless, the percentages of alterations were substantial (− 61%, − 207%, − 71%, + 41% respectively) indicating remarkable burst activity suppression by 2-DG (N = 3 in epileptic and epileptic + 2-DG groups)
Histology
Kainic Acid Led to Severe Pyramidal and NADPH-d Positive Cell Loss in the Dorsal CA1 Pyramidal Layer
Nissl staining showed that the integrity of the dorsal CA1 pyramidal cell layer was disrupted and remarkable cell loss, especially in CA1, was evident following intrahippocampal kainic acid administration (Fig. 2C, D). In parallel, NADPH histochemical staining for nitrergic neurons revealed that the number of NADPH-d+ cells in the pyramidal layer of dorsal CA1was significantly decreased (P < 0.05) in the epileptic (N = 3 mice, 21 sections) animals when compared with the control group (N = 4 mice, 28 sections) and epileptic (N = 3 mice, 21 sections) groups (Fig. 8A, a, B, b, C, c, D, d, E).
Distribution of NADPH diaphorase positive neurons in pyramidal cell layer of dorsal CA1 in control A, a, control + 2-DG B, b, epileptic C, c, and epileptic + 2-DG D, d groups. 2-DG led to a significant increase in the number of NADPH-d+ neurons in the control + 2-DG group (N = 4) compared to the control (N = 4) group while failed to reverse severe NADPH-d+ decrement induced by epilepsy induction (N = 3 and 4 in the epileptic and epileptic + 2-DG groups, respectively) E. In the contralateral dorsal hippocampus, however, NADPH-d+ cells were significantly higher compared to the ipsilateral dorsal hippocampus both in epileptic F and epileptic + 2-DG G groups. Note that the number of NADPH-d+ neurons is slightly higher in the contralateral CA1 of epileptic and epileptic + 2DG animals compared to the control animals. The NADPH-d+ cells are shown by the arrows. Data are shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars: A 300 µm, B 100 µm
2-DG Treatment Increased NADPH-d+ Neurons in the Dorsal Hippocampus of Control Animals While Failing to Alter their Number in the Kainic Acid-Treated Animals
In the control + 2-DG group, elevated NADPH-d+ was observed compared with the control group (20.71 ± 1.01 in the control + 2DG group (N = 4 mice, 28 sections); p-value < 0.001; Fig. 8E). In the epileptic group receiving 2-DG, however, no significant alteration in the number of NADPH-d-stained neurons (N = 4 mice, 28 sections; p-value = 0.73; Fig. 8E) was noted compared with the epileptic group. In contrast, in the contralateral dorsal hippocampus of epileptic and the epileptic + 2-DG group, the NADPH-d+ cell number was approximately threefold more in comparison with the ipsilateral side (P < 0.05; Fig. 8F, G); Note that the number of NADPH-d+ neurons in the contralateral hippocampus of these groups was even mildly more compared to control group. There was no notable difference between the ipsilateral and contralateral hippocampus in the control group (data not shown).
Discussion
In the present study, we aimed to ascertain the extent to which glycolysis inhibition by 2-DG affects cellular function, electrophysiological measures, and epilepsy-related behavioral deficits using the mouse intrahippocampal kainic acid model. The unilateral intrahippocampal kainic acid model is a relevant animal model of medial temporal lobe epilepsy and provides a useful platform to investigate the mechanisms of epilepsy and the effectiveness of therapies for temporal lobe epilepsy, including inhibition of glycolysis by 2-DG. Its anticonvulsant efficacy in several rodent models of epilepsy, such as chemoconvulsant [33, 34] and electroconvulsive [35] seizure models has been demonstrated.
In this study, we used behavioral, electrophysiological and histochemical approaches to elucidate the impact of 2-DG treatment on alterations induced by intrahippocampal microinjection of kainic acid. Here, all experiments exclusively were conducted on male mice for the following reasons. First, there is agreement among studies that males have a higher incidence of epilepsy and they are more vulnerable to epilepsy acquisition than females in human populations [36, 37], and second, the difference in the fluctuation of the hormone levels between females during the ovarian cycles might differentially cause variability in the data [38, 39], thus making interpretation of comparison more difficult.
Our behavioral results showed a decrease in the anxiety behavior measured by the zero-maze test, but not the open field test, as well as an increase in the locomotor activity and depression behavior in the epileptic animals. It has been elucidated that there is a link between hippocampal sclerosis and depression-like behavior in epileptic models [15, 16]. In our model of temporal lobe epilepsy, hippocampal sclerosis is a well-known remark, as evidenced by a significant cell loss in the CA1 area of the hippocampus. This is consistent with the finding reported by [40, 41]. Although a previous study showed no depression behavior in the mice model of intrahippocampal kainic acid using tail suspension and forced swimming tests [14], here, we report a significant depression behavior in the NMRI mice model of epilepsy-induced by intrahippocampal injection of kainic acid, as evidenced by a significant reduction in the preference for the 3% sucrose solution compared to control mice. This finding is consistent with previous studies in epileptic mice [31, 42]. The sucrose preference test was used as a measure of anhedonia, which is the core symptom of depression and it is a behavior that is commonly observed in patients with the major depressive disorder [43].
Moreover, hippocampal lesions reduce anxiety behavior in rodents. Even so, a considerable controversy is faced when it comes to differentiating behavioral disorders, including anxiety, between the dorsal and/or ventral hippocampus of lesioned animals [44, 45]. Locomotor activity, however, is affected by such lesions especially dorsal hippocampus lesions [46]. Increased locomotor activity has already been reported in the intrahippocampal kainic acid model of temporal lobe epilepsy [14]; the same study also reported no altered anxiety behavior in kainic acid-treated mice in the elevated plus-maze test.
Nonetheless, here, we explicitly show that both locomotor activity and anxiety behavior are altered in the epileptic group, as evidenced by increased locomotor activity and decreased anxiety behavior. Although cell loss is noted in the ventral hippocampus besides the dorsal part (kainic acid injection site), sclerosis is mostly seen in the dorsal hippocampus [47]. This fact raises a hypothesis about whether increased locomotion due to dorsal hippocampal sclerosis has affected the zero-maze parameters. To test this hypothesis, we first attempted to see if there was a correlation between the locomotion state and each parameter of the zero-maze test. Interestingly, all the parameters except for body stretching frequency were significantly correlated to the locomotion state of the animals. Hence, using the ANCOVA test, we masked locomotion’s effect on the parameters and the results were staggering; except for head dipping frequency, the other three parameters (open-arm time, number of open-arm entry, and latency of first open arm entry) did not reach to significance level between the three groups while ANOVA test results were indicating quite the opposite. All in all, at least to some extent, significantly reduced anxiety- like behavior in the kainic acid-treated animals compared with the control animals, was a result of augmented locomotor activity. Consistently, body stretching is deemed to be the most emotionally-driven posture of the animal in the zero-maze apparatus [30]; here we indicated that body stretching frequency is not affected by locomotion state and is not significantly different between the three groups. Masking interventional effect of increased locomotion on zero-maze results, unchanged anxiety behavior in epileptic animals was noted; this was consistent with unchanged anxiety extrapolated from thigmotaxis assessment. Maia et al. have shown that rats treated with kainic acid were hyperactive in the open-field test and exhibited less anxiety-like behaviors [48]. They have also reported that kainic acid treatment was associated with severe cell loss in hippocampus. These findings are in line with our results.
In the present study, we showed that 2-DG at the cellular level almost suppressed the electrophysiological alterations induced by intrahippocampal injection of kainic acid, which produces one of the most drug-resistant epilepsy models with a striking resemblance to human epilepsy because of sclerosis seen in the hippocampus of the treated animals. However, at the neuronal circuit level, 2-DG seems to fail to suppress the sharp-wave complexes. This incident begs the question of whether neuronal electrophysiological alterations induced by kainic acid injection are responsible for this incompetency of 2-DG. In this regard, Forte et al. [49] illuminated that 2-DG exerts its anticonvulsant effects through different mechanisms at the cellular and circuit levels; at the cellular level, KATP channels seem to play the major role while at the circuit level, suppression of epileptiform activity depends on GABA-A receptor activation. This activation is mediated by increased pentose phosphate pathway (ppp) as glycolysis enzyme phospho-fructo kinase is inhibited by 2-DG, and subsequently, upstream substrates are shifted to ppp.
Even though inhibited IPSPs are postulated to be the primary mechanism of hyperexcitability in surviving CA1 pyramidal neurons [17, 50], analysis of passive properties of CA1 neurons showed decreased membrane capacitance which leads to membrane tau decrement and hyperexcitability. This capacitance decrease is likely to occur due to diminished cell size seen in the intrahippocampal kainic acid-treated animals as we demonstrated here. However, consistent with the previous reports, 2-DG did not alter the passive membrane properties of CA1 pyramidal neurons [7]. Furthermore, alterations in the active properties of CA1 pyramidal neurons in the epileptic mice were associated with a decrease in the rebound AP latency following a hyperpolarizing current which is evidence of neuronal hyperexcitability [51]. However, here, we revealed that 2-DG can substantially reduce the firing rate in epileptic animals. Since inhibition of glycolysis is associated with ATP deprivation, there is posited to be a link between firing rate alteration following 2-DG injection and subsequent KATP channel [52]. One probable explanation for the reduction in neuronal excitability induced by the 2-DG treatment could be the prolonged AP duration and subsequent relative refractory period increment, which is likely to be a key player in burst activity suppression and decreased AP frequency throughout the recording.
Moreover, our findings showed a decrease in the AP rising tau as well as an increase in the amplitude and duration of APs in the 2-DG- treated epileptic mice. Since inhibition of glycolysis by 2-DG could be associated with attenuation of ATP level in the neurons, this, in turn, may affect the function of ion channels and/or Na+-K+ ATPase that are involved in AP production and thereby changes the AP waveform. Since there is a tight coupling between Na+-K+ pump and glycolysis [53], its functional disruption causes severe alteration in neuronal activity [54,55,56]. Furthermore, inhibition of glycolysis by 2-DG has also been reported to suppress synaptic transmission in the CA1 region of hippocampus [57].
Next, we assessed whether induction of epileptic activity and glycolysis inhibition modify the NADPH-diaphorase activity, as a histochemical marker of the nitric oxide synthase (NOS), since there are several reports confirming the role of the nitric oxide system in the pathophysiology of mood disorders, including depression [58, 59], and epilepsy [60,61,62]. A reduced number of NADPH-d+ cells in the dorsal hippocampus of i.p and i.c.v kainic-acid treated animals has already been reported [22, 63]. Here we show that NADPH-d+ cells are smaller in number in the ipsilateral dorsal hippocampus CA1 in the intrahippocampal kainic acid model of epilepsy. Consistent with previous reports [64] however, in the contralateral dorsal CA1 of epileptic animals (and also epileptic + 2-DG group), NADPH-d+ cells were slightly more frequent than in the control animals. This could be a compensatory mechanism attempting to augment NO signaling in the contralateral hippocampus after decreased NADPH-d+ cell number in the kainic acid-treated side. Although 2-DG was unable to increase NADPH-d+ cell number in the drug animals, it led to an increase in the number of NADPH-d+ in control-2-DG received rats. It could be speculated that increased NADPH concentration in the interneurons owing to pentose phosphate pathway (PPP) potentiation following glycolysis inhibition by 2-DG leads to such augmentation. To explain more, increased NADPH will bring about increased reduction of nitro blue tetrazolium to diformazan (the visible dye) inside the neurons containing NADPH diaphorase which were not NADPH-d+ when PPP and, subsequently NADPH concentration was low. Interestingly, NADPH-d+ neurons in the hippocampus are demonstrated to release GABA too, indicating that NO acts as a paracrine/retrograde co-transmitter [65]. If so, loss of these GABAergic neurons could be a cause of disappeared IPSPs following the kainic acid injection, mentioned above. Additionally, it is recently argued that NADPH diaphorase activity in aldehyde-fixed tissue is not enzymatic rather, it is mediated by NO-containing factors which promote the reduction of nitro blue tetrazolium to diformazan [66].
In conclusion, while, at the cellular level, 2-DG treatment significantly reverses the electrophysiological alterations following epilepsy induction by intrahippocampal kainic acid injection, it seems to be incompetent in suppressing circuit-level changes (as shown by interictal epileptiform activity). In behavioral part of our study, on the other hand, only partial improvement was noted which could be a direct impression of hypometabolism induced by 2-DG. Moreover, while glycolysis inhibition by 2-DG was associated with an increase in the number of NADPH-d+ cells in the control group, its application was unable to alter the NADPH-diaphorase activity, as did not change the number of NADPH-d+ neurons in the epileptic animals, which may imply a severe NADPH-d+ cell loss.
Limitation of the Present Study
It should be noted that due to severe cell loss in CA1, finding healthy neurons was a daunting task as the remaining neurons were rather fragile. Consequently, the sample size is rather small (see below). Even so, for the first time, as far as we know, in the intrahippocampal kainic acid model, we report the passive properties of the cell membrane and action potential (AP) properties of the dorsal CA1 pyramidal neurons, using the whole-cell patch-clamp recording. Furthermore, since in the present study we have not done voltage-clamp recordings of ion channels, the discussion on the cellular basis of altered neuronal excitability is speculative.
Data Availability
Data are available from the corresponding author on reasonable request.
References
Boison D, Steinhäuser C (2018) Epilepsy and astrocyte energy metabolism. Glia 66(6):1235–1243. https://doi.org/10.1002/glia.23247
Sada N, Lee S, Katsu T, Otsuki T, Inoue T (2015) Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 347(6228):1362–1367. https://doi.org/10.1126/science.aaa1299
Nedergaard S, Andreasen M (2018) Opposing effects of 2-deoxy-d-glucose on interictal- and ictal-like activity when K+ currents and GABA A receptors are blocked in rat hippocampus in vitro. J Neurophysiol 119(5):1912–1923. https://doi.org/10.1152/jn.00732.2017
Leiter I, Bascuñana P, Bengel FM, Bankstahl JP, Bankstahl M (2018) (2019) “Attenuation of epileptogenesis by 2-deoxy-d-glucose is accompanied by increased cerebral glucose supply, microglial activation and reduced astrocytosis”. Neurobiol Dis 130(September):104510. https://doi.org/10.1016/j.nbd.2019.104510
Gasior M, Yankura J, Hartman AL, French A, Rogawski MA (2010) Anticonvulsant and proconvulsant actions of 2-deoxy-d-glucose. Epilepsia 51(8):1385–1394. https://doi.org/10.1111/j.1528-1167.2010.02593.x
Yang H, Guo R, Wu J, Peng Y, Xie D, Zheng W, Huang X (2013) The antiepileptic effect of the glycolytic inhibitor 2-deoxy-d-glucose is mediated by upregulation of KATP channel subunits Kir6.1 and Kir6.2. Neurochem Res 38(4):677–685. https://doi.org/10.1007/s11064-012-0958-z
Shao LR, Stafstrom CE (2017) Glycolytic inhibition by 2-deoxy-d-glucose abolishes both neuronal and network bursts in an in vitro seizure model. J Neurophysiol 118(1):103–113. https://doi.org/10.1152/jn.00100.2017
Lian XY, Khan FA, Stringer JL (2007) Fructose-1,6-bisphosphate has anticonvulsant activity in models of acute seizures in adult rats. J Neurosci 27(44):12007–12011. https://doi.org/10.1523/JNEUROSCI.3163-07.2007
Stafstrom CE, Ockuly JC, Murphree L, Valley MT, Roopra A, Sutula TP (2009) Anticonvulsant and antiepileptic actions of 2-deoxy-d-glucose in epilepsy models. Ann Neurol 65(4):435–447. https://doi.org/10.1002/ana.21603
Karangula J, Boggula N, Kappari V (2021) 2-Deoxy-d-glucose: an update review. J Innov Dev Pharm Tech Sci 4(5):68–78
Goel R (2021) 2-Deoxy-d-glucose: from diagnostics to therapeutics. Int J Basic Clin Pharmacol 10(6):732. https://doi.org/10.18203/2319-2003.ijbcp20212086
Lothman EW, Collins RC (1981) Kainic acid induced limbic seizures: metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res 218(1–2):299–318. https://doi.org/10.1016/0006-8993(81)91308-1
French ED, Aldinio C, Schwarcz R (1982) Intrahippocampal kainic acid, seizures and local neuronal degeneration: relationships assessed in unanesthetized rats. Neuroscience 7(10):2525–2536. https://doi.org/10.1016/0306-4522(82)90212-3
Gröticke I, Hoffmann K, Löscher W (2008) Behavioral alterations in a mouse model of temporal lobe epilepsy induced by intrahippocampal injection of kainate. Exp Neurol 213(1):71–83. https://doi.org/10.1016/j.expneurol.2008.04.036
Bannerman DM et al (2004) Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev 28(3):273–283. https://doi.org/10.1016/j.neubiorev.2004.03.004
Quiske A, Helmstaedter C, Lux S, Elger CE (2000) Depression in patients with temporal lobe epilepsy is related to mesial temporal sclerosis. Epilepsy Res 39(2):121–125. https://doi.org/10.1016/S0920-1211(99)00117-5
Duveau V, Pouyatos B, Bressand K, Bouyssières C, Chabrol T, Roche Y, Depaulis A, Roucard C (2016) Differential effects of antiepileptic drugs on focal seizures in the intrahippocampal kainate mouse model of mesial temporal lobe epilepsy. CNS Neurosci Ther 22(6):497–506. https://doi.org/10.1111/cns.12523
Turner DA, Wheal HV (1991) Excitatory synaptic potentials in kainic acid-denervated rat CA1 pyramidal neurons. J Neurosci 11(9):2786–2794. https://doi.org/10.1523/jneurosci.11-09-02786.1991
Franck JE, Kunkel DD, Baskin DG, Schwartzkroin PA (1988) Inhibition in kainate-lesioned hyperexcitable hippocampi: physiologic, autoradiographic, and immunocytochemical observations. J Neurosci 8(6):1991–2002. https://doi.org/10.1523/jneurosci.08-06-01991.1988
Scheggi S, De Montis MG, Gambarana C (2018) Making sense of rodent models of anhedonia. Int J Neuropsychopharmacol 21(11):1049–1065. https://doi.org/10.1093/ijnp/pyy083
Liu MY, Yin C, Zhu L, Zhu X, Xu C, Luo C et al (2018) Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc 13(7):1686–1698. https://doi.org/10.1038/s41596-018-0011-z
Dudina YV (2005) Effect of kainate-induced experimental epilepsy on NADPH-diaphorase and calcium-binding proteins in rat hippocampal neurons. Bull Exp Biol Med 139(3):309–312. https://doi.org/10.1007/s10517-005-0279-1
Riljak V, Milotová M, Jandová K, Pokorný J, Langmeier M (2007) Morphological changes in the hippocampus following nicotine and kainic acid administration. Physiol Res 56(5):641–649. https://doi.org/10.33549/physiolres.931048
Neely C, Lane C, Torres J, Flinn J (2018) “The effect of gentle handling on depressive-like behavior in adult male mice: considerations for human and rodent interactions in the laboratory”, 2018. Behav Neurol. https://doi.org/10.1155/2018/2976014
Sensini F, Inta D, Palme R et al (2020) The impact of handling technique and handling frequency on laboratory mouse welfare is sex-specific. Sci Rep 10:17281. https://doi.org/10.1038/s41598-020-74279-3
Buckinx A, Van Schuerbeek A, Bossuyt J, Allaoui W, Van Den Herrewegen Y, Smolders I, De Bundel D (2021) Exploring refinement strategies for single housing of male C57BL/6JRj mice: effect of cage divider on stress-related behavior and hypothalamic-pituitary-adrenal-axis activity. Front Behav Neurosci 28(15):743959. https://doi.org/10.3389/fnbeh.2021.743959
Koenig JB, Cantu D, Low C, Sommer M, Noubary F, Croker D, Whalen M, Kong D, Dulla CG (2019) Glycolytic inhibitor 2-deoxyglucose prevents cortical hyperexcitability after traumatic brain injury. JCI Insight 5(11):e126506. https://doi.org/10.1172/jci.insight.126506
Ghotbedin Z, Janahmadi M, Mirnajafi-Zadeh J, Behzadi G, Semnanian S (2013) Electrical low frequency stimulation of the kindling site preserves the electrophysiological properties of the rat hippocampal CA1 pyramidal neurons from the destructive effects of amygdala kindling: the basis for a possible promising epilepsy therapy. Brain Stimul 6(4):515–523. https://doi.org/10.1016/J.BRS.2012.11.001
Tucker LB, McCabe JT (2017) Behavior of male and female C57Bl/6J mice is more consistent with repeated trials in the elevated zero maze than in the elevated plus maze. Front Behav Neurosci 11:1–8. https://doi.org/10.3389/fnbeh.2017.00013
Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT (1994) Behavioural and pharmacological characterisation of the elevated ‘zero-maze’ as an animal model of anxiety. Psychopharmacology 116(1):56–64. https://doi.org/10.1007/BF02244871
Klein S, Bankstahl JP, Löscher W, Bankstahl M (2015) Sucrose consumption test reveals pharmacoresistant depression-associated behavior in two mouse models of temporal lobe epilepsy. Exp Neurol 263:263–271. https://doi.org/10.1016/j.expneurol.2014.09.004
Su H, Sochivko D, Becker A, Chen J, Jiang Y, Yaari Y, Beck H (2002) Upregulation of a t-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J Neurosci 22(9):3645–3655. https://doi.org/10.1523/jneurosci.22-09-03645.2002
Shao LR, Rho JM, Stafstrom CE (2018) Glycolytic inhibition: a novel approach toward controlling neuronal excitability and seizures. Epilepsia Open 3(2):191. https://doi.org/10.1002/EPI4.12251
Rho JM, Shao LR, Stafstrom CE (2019) 2-Deoxyglucose and beta-hydroxybutyrate: metabolic agents for seizure control. Front Cell Neurosci. https://doi.org/10.3389/FNCEL.2019.00172
Wijayasinghe YS, Bhansali MP, Borkar MR, Chaturbhuj GU, Muntean BS, Viola RE, Bhansali PR (2022) A comprehensive biological and synthetic perspective on 2-deoxy-d-glucose (2-DG), a sweet molecule with therapeutic and diagnostic potentials. J Med Chem 65(5):3706–3728. https://doi.org/10.1021/acs.jmedchem.1c01737
McHugh JC, Delanty N (2008) Epidemiology and classification of epilepsy: gender comparisons. Int Rev Neurobiol 83:11–26. https://doi.org/10.1016/S0074-7742(08)00002-0
Hauser WA, Annegers JF, Kurland LT (1991) Prevalence of epilepsy in Rochester, Minnesota: 1940–1980. Epilepsia 32(4):429–445. https://doi.org/10.1111/J.1528-1157.1991.TB04675.X
Velíšková J, DeSantis KA (2013) Sex and hormonal influences on seizures and epilepsy. Horm Behav 63(2):267–277. https://doi.org/10.1016/J.YHBEH.2012.03.018
Taubøll E, Sveberg L, Svalheim S (2015) Interactions between hormones and epilepsy. Seizure 28:3–11. https://doi.org/10.1016/J.SEIZURE.2015.02.012
Maroso M, Balosso S, Ravizza T, Iori V, Wright C, French J, Vezzani A (2011) Interleukin-1β biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics 8(2):304–315. https://doi.org/10.1007/s13311-011-0039-z
Hokkanen SRK, Hunter S, Polvikoski TM, Keage HAD, Minett T, Matthews FE, Brayne C, MRC CFAS and CC75C Study Group (2018) Hippocampal sclerosis, hippocampal neuron loss patterns and TDP-43 in the aged population. Brain Pathol 28(4):548–559. https://doi.org/10.1111/bpa.12556
Oliveira CV, Grigoletto J, Funck VR, Ribeiro LR, Royes LFF, Fighera MR et al (2015) Evaluation of potential gender-related differences in behavioral and cognitive alterations following pilocarpine-induced status epilepticus in C57BL/6 mice. Physiol Behav 143:142–150. https://doi.org/10.1016/j.physbeh.2015.03.004
Wulsin AC, Franco-Villanueva A, Romancheck C, Morano RL, Smith BL, Packard BA, Danzer SC, Herman JP (2018) Functional disruption of stress modulatory circuits in a model of temporal lobe epilepsy. PLoS ONE 13(5):e0197955. https://doi.org/10.1371/journal.pone.0197955
Park C, Kang M, Kang K, Lee J, Kim J, Yoo J, Ahn H, Huh Y (2001) Differential changes in neuropeptide Y and nicotinamide adenine dinucleotide phosphate-diaphorase-positive neurons in rat hippocampus after kainic acid-induced seizure. Neurosci Lett 298(1):49–52. https://doi.org/10.1016/s0304-3940(00)01720-1
Zhang W, Bast T, Xu Y, Feldon J (2014) Temporary inhibition of dorsal or ventral hippocampus by muscimol: distinct effects on measures of innate anxiety on the elevated plus maze, but similar disruption of contextual fear conditioning. Behav Brain Res 262:47–56. https://doi.org/10.1016/j.bbr.2013.10.044
Bast T, Feldon J (2003) Hippocampal modulation of sensorimotor processes. Prog Neurobiol 70(4):319–345. https://doi.org/10.1016/S0301-0082(03)00112-6
Le Duigou C, Bouilleret V, Miles R (2008) Epileptiform activities in slices of hippocampus from mice after intra-hippocampal injection of kainic acid. J Physiol 586(20):4891–4904. https://doi.org/10.1113/jphysiol.2008.156281
Maia GH, Quesado JL, Soares JI, do Carmo JM, Andrade PA, Andrade JP, Lukoyanov NV (2014) Loss of hippocampal neurons after kainate treatment correlates with behavioral deficits. PLoS ONE 9(1):e84722. https://doi.org/10.1371/journal.pone.0084722
Forte N, Medrihan L, Cappetti B, Baldelli P, Benfenati F (2016) 2-Deoxy-d-glucose enhances tonic inhibition through the neurosteroid-mediated activation of extrasynaptic GABA A receptors. Epilepsia. https://doi.org/10.1111/epi.13578
Bouilleret V, Loup F, Kiener T, Marescaux C, Fritschy JM (2000) Early loss of interneurons and delayed subunit-specific changes in GABA(A)-receptor expression in a mouse model of mesial temporal lobe epilepsy. Hippocampus 10(3):305–324. https://doi.org/10.1002/1098-1063(2000)10:3%3c305::AID-HIPO11%3e3.0.CO;2-I
Chen Z, Ljunggren HG, Bogdanovic N, Nennesmo I, Winblad B, Zhu J (2002) Excitotoxic neurodegeneration induced by intranasal administration of kainic acid in C57BL/6 mice. Brain Res 931(2):135–145. https://doi.org/10.1016/S0006-8993(02)02268-0
Lutas A, Yellen G (2013) The ketogenic diet : metabolic influences on brain excitability and epilepsy. Trends Neurosci 36(1):32–40. https://doi.org/10.1016/j.tins.2012.11.005
Erecińska M, Dagani F, Nelson D, Deas J, Silver IA (1991) Relations between intracellular ions and energy metabolism: a study with monensin in synaptosomes, neurons, and C6 glioma cells. J Neurosci 11(8):2410–2421. https://doi.org/10.1523/JNEUROSCI.11-08-02410.1991
Reich CG, Mason SE, Alger BE (2004) Novel form of LTD induced by transient, partial inhibition of the Na, K-pump in rat hippocampal CA1 cells. J Neurophysiol 91:239–247
Kumar AR, Kurup PA (2002) Inhibition of membrane Na+-K+ ATPase activity: a common pathway in central nervous system disorders. J Assoc Physicians India 50:400–406
Anderson TR, Huguenard JR, Prince DA (2010) Differential effects of Na+-K+ ATPase blockade on cortical layer V neurons. J Physiol 588(Pt 22):4401–4414. https://doi.org/10.1113/jphysiol.191858
Zhao YT, Tekkök S, Krnjević K (1997) 2-Deoxy-d-glucose-induced changes in membrane potential, input resistance, and excitatory postsynaptic potentials of CA1 hippocampal neurons. Can J Physiol Pharmacol 75(5):368–374
Ghasemi M, Claunch J, Niu K (2018) Pathologic role of nitrergic neurotransmission in mood disorders. Prog Neurobiol. https://doi.org/10.1016/j.pneurobio.2018.06.002
Ghasemi M (2019) “Nitric oxide : antidepressant mechanisms and inflammation. Adv Pharmacol 86:121–152. https://doi.org/10.1016/bs.apha.2019.04.004
Borowicz KK, Stanisław R (2020) Role of oxidative stress in epileptogenesis and potential implications for therapy. Pharmacol Reports 72(5):1218–1226. https://doi.org/10.1007/s43440-020-00143-w
Rabanus A, Ota J, Patzak A, Kardos J, Albus K, Heinemann U, Kann O (2009) Endogenous nitric oxide is a key promoting factor for initiation of seizure-like events in hippocampal and entorhinal cortex slices. J Neurosci 29(26):8565–8577. https://doi.org/10.1523/JNEUROSCI.5698-08.2009
Banach M, Piskorska B, Czuczwar SJ, Borowicz KK (2011) “Nitric oxide, epileptic seizures, and action of antiepileptic drugs. CNS Neurol Disord 10(7):808–819
Jose CD, Megias M, Andreu MJ, Real MA, Guirado S (1995) NADPH diaphorase-positive neurons in the lizard hippocampus: a distinct subpopulation of gabaergic interneurons. Hippocampus 70(5):60–70
Yasuda H, Fujii M, Fujisawa H, Ito H, Suzuki M (2001) Changes in nitric oxide synthesis and epileptic activity in the contralateral hippocampus of rats following intrahippocampal kainate injection. Epilepsia 42(1):13–20. https://doi.org/10.1046/j.1528-1157.2001.083032.x
Reis PA, Gutierrez TM, Silva AR, Caire H, Faria DC (2017) Role of nitric oxide synthase in the function of the central nervous system under normal and infectious conditions. Nitric oxide synthase—simple enzyme-complex roles. IntechOpen, London
Seckler JM, Shen J, Lewis T, Abdulamir M, Zaman K, Palmer L, Bates J, Jenkins M, Lewis S (2020) NADPH diaphorase detects S—nitrosylated proteins in aldehyde—treated biological tissues. Sci Rep. https://doi.org/10.1038/s41598-020-78107-6
Funding
This work was supported financially by Elite Researcher Grant Committee under award numbers 971414 and 996644 from National Institute for Medical Research Development (NIMAD, Grant No. 996644) and in part by Shahid Beheshti University of Medical Sciences (Grant No. 24402), Tehran, Iran.
Author information
Authors and Affiliations
Contributions
K and R: carried out the experiments, analyzed the data and wrote the draft of the article. R, D, N, M: analyzed the data, wrote the MATLAB codes. R, M, B and H: helped supervise the project and critically reviewed the article. J: conceived, designed the experiments, supervised the research, wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Conflict of interest
All authors have been substantially involved in the preparation of the present manuscript and no undisclosed groups or persons have had a primary role in the study. All authors have seen and approved the submitted version of the paper and accept responsibility for its content. The authors declare no conflict of interest and declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics Approval
All experimental procedures and animal care were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Biomedical Research Ethics Committee of the National Institute for Medical Research Development (Approval ID: IR.NIMAD.REC.1399.259) and the Ethics Committee of Shahid Beheshti University of Medical Sciences (Authorization code: IR.SBMU.MSP.REC.1400.630).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khatibi, V.A., Rahdar, M., Rezaei, M. et al. The Glycolysis Inhibitor 2-Deoxy-d-Glucose Exerts Different Neuronal Effects at Circuit and Cellular Levels, Partially Reverses Behavioral Alterations and does not Prevent NADPH Diaphorase Activity Reduction in the Intrahippocampal Kainic Acid Model of Temporal Lobe Epilepsy. Neurochem Res 48, 210–228 (2023). https://doi.org/10.1007/s11064-022-03740-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03740-8