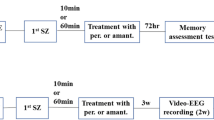
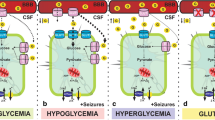
Abstract
Status epilepticus (SE) is defined as continuous and self-sustaining seizures, which trigger hippocampal neurodegeneration, mitochondrial dysfunction, oxidative stress, and energy failure. During SE, the neurons become overexcited, increasing energy consumption. Glucose uptake is increased via the sodium glucose cotransporter 1 (SGLT1) in the hippocampus under epileptic conditions. In addition, modulation of glucose can prevent neuronal damage caused by SE. Here, we evaluated the effect of increased glucose availability in behavior of limbic seizures, memory dysfunction, neurodegeneration process, neuronal activity, and SGLT1 expression. Vehicle (VEH, saline 0.9%, 1 μL) or glucose (GLU; 1, 2 or 3 mM, 1 μL) were administered into hippocampus of male Wistar rats (Rattus norvegicus) before or after pilocarpine to induce SE. Behavioral analysis of seizures was performed for 90 min during SE. The memory and learning processes were analyzed by the inhibitory avoidance test. After 24 h of SE, neurodegeneration process, neuronal activity, and SGLT1 expression were evaluated in hippocampal and extrahippocampal regions. Modulation of hippocampal glucose did not protect memory dysfunction followed by SE. Our results showed that the administration of glucose after pilocarpine reduced the severity of seizures, as well as the number of limbic seizures. Similarly, glucose after SE reduced cell death and neuronal activity in hippocampus, subiculum, thalamus, amygdala, and cortical areas. Finally, glucose infusion elevated the SGLT1 expression in hippocampus. Taken together our data suggest that possibly the administration of intrahippocampal glucose protects brain in the earlier stage of epileptogenic processes via an important support of SGLT1.







Similar content being viewed by others
References
Sloviter RS (1999) Status epilepticus-induced neuronal injury and network reorganization. Epilepsia 40:34–39. https://doi.org/10.1111/j.1528-1157.1999.tb00876.x
Sánchez S, Rincon F (2016) Status epilepticus: epidemiology and public health needs. J Clin Med 5. https://doi.org/10.3390/jcm5080071
Lowenstein DH, Bleck T, Macdonald RL (1999) It’s time to revise the definition of status epilepticus. Epilepsia 40:120–122
Santos VR, Melo IS, Pacheco ALD, Castro OW (2019) Life and death in the hippocampus : what’ s bad ? Epilepsy Behav 106595:106595. https://doi.org/10.1016/j.yebeh.2019.106595
Upadhya D, Castro OW, Upadhya R, Shetty AK (2018) Prospects of cannabidiol for easing status epilepticus-induced epileptogenesis and related comorbidities. Mol Neurobiol 55:6956–6964. https://doi.org/10.1007/s12035-018-0898-y
Castro OW, Upadhya D, Kodali M, Shetty AK (2017) Resveratrol for easing status epilepticus induced brain injury, inflammation, epileptogenesis, and cognitive and memory dysfunction—are we there yet? Front Neurol 8:603. https://doi.org/10.3389/fneur.2017.00603
De Furtado MA, Braga GK, Oliveira JAC et al (2002) Behavioral, morphologic, and electroencephalographic evaluation of seizures induced by intrahippocampal microinjection of pilocarpine. Epilepsia 43:37–39. https://doi.org/10.1046/j.1528-1157.2002.043s2037.x
Kälviäinen R, Reinikainen M (2019) Management of prolonged epileptic seizures and status epilepticus in palliative care patients. Epilepsy Behav 101:106288. https://doi.org/10.1016/j.yebeh.2019.04.041
Ho C-J, Lin C-H, Lu Y-T, Shih FY, Hsu CW, Tsai WC, Tsai MH (2019) Perampanel treatment for refractory status epilepticus in a neurological intensive care unit. Neurocrit Care 31:24–29. https://doi.org/10.1007/s12028-019-00704-9
Trinka E, Kälviäinen R (2017) 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure 44:65–73. https://doi.org/10.1016/j.seizure.2016.11.001
Furtado MA, Castro OW, Del Vecchio F et al (2011) Study of spontaneous recurrent seizures and morphological alterations after status epilepticus induced by intrahippocampal injection of pilocarpine. Epilepsy Behav 20:257–266. https://doi.org/10.1016/j.yebeh.2010.11.024
Castro OW, Furtado MA, Tilelli CQ et al (2011) Comparative neuroanatomical and temporal characterization of FluoroJade-positive neurodegeneration after status epilepticus induced by systemic and intrahippocampal pilocarpine in Wistar rats. Brain Res 1374:43–55. https://doi.org/10.1016/j.brainres.2010.12.012
Melo IS, Santos YMO, Costa MA, Pacheco ALD, Silva NKGT, Cardoso-Sousa L, Pereira UP, Goulart LR et al (2016) Inhibition of sodium glucose cotransporters following status epilepticus induced by intrahippocampal pilocarpine affects neurodegeneration process in hippocampus. Epilepsy Behav 61:258–268. https://doi.org/10.1016/j.yebeh.2016.05.026
Rodrigues MCA, Rossetti F, Foresti ML, Arisi GM, Furtado MA, Dal-Cól MLC, Bertti P, Fernandes A et al (2005) Correlation between shaking behaviors and seizure severity in five animal models of convulsive seizures. Epilepsy Behav 6:328–336. https://doi.org/10.1016/j.yebeh.2005.02.005
Lai M-C, Lin K-M, Yeh P-S, Wu SN, Huang CW (2018) The novel effect of immunomodulator-glatiramer acetate on epileptogenesis and epileptic seizures. Cell Physiol Biochem 50:150–168. https://doi.org/10.1159/000493965
Wu Q, Wang H (2018) The spatiotemporal expression changes of CB2R in the hippocampus of rats following pilocarpine-induced status epilepticus. Epilepsy Res 148:8–16. https://doi.org/10.1016/j.eplepsyres.2018.10.002
Oliveira TL, Candeia-Medeiros N, Cavalcante-Araújo PM, Melo IS, Fávaro-Pípi E, Fátima LA, Rocha AA, Goulart LR et al (2016) SGLT1 activity in lung alveolar cells of diabetic rats modulates airway surface liquid glucose concentration and bacterial proliferation. Sci Rep 6:21752. https://doi.org/10.1038/srep21752
Jung K-H, Chu K, Lee S-T, Kim JH, Kang KM, Song EC, Kim SJ, Park HK et al (2009) Region-specific plasticity in the epileptic rat brain: a hippocampal and extrahippocampal analysis. Epilepsia 50:537–549. https://doi.org/10.1111/j.1528-1167.2008.01718.x
Zenki KC, Kalinine E, Zimmer ER, dos Santos TG, Mussulini BHM, Portela LVC, de Oliveira DL (2018) Memantine decreases neuronal degeneration in young rats submitted to LiCl-pilocarpine-induced status epilepticus. Neurotoxicology 66:45–52. https://doi.org/10.1016/j.neuro.2018.03.005
Scholl EA, Dudek FE, Ekstrand JJ (2013) Neuronal degeneration is observed in multiple regions outside the hippocampus after lithium pilocarpine-induced status epilepticus in the immature rat. Neuroscience 252:45–59. https://doi.org/10.1016/j.neuroscience.2013.07.045
Loss CM, Córdova SD, de Oliveira DL (2012) Ketamine reduces neuronal degeneration and anxiety levels when administered during early life-induced status epilepticus in rats. Brain Res 1474:110–117. https://doi.org/10.1016/j.brainres.2012.07.046
Santos LFL, Freitas RLM, Xavier SML, Saldanha GB, Freitas RM (2008) Neuroprotective actions of vitamin C related to decreased lipid peroxidation and increased catalase activity in adult rats after pilocarpine-induced seizures. Pharmacol Biochem Behav 89:1–5. https://doi.org/10.1016/j.pbb.2007.10.007
dos Santos PS, Costa JP, Tomé ADR et al (2011) Oxidative stress in rat striatum after pilocarpine-induced seizures is diminished by alpha-tocopherol. Eur J Pharmacol 668:65–71. https://doi.org/10.1016/j.ejphar.2011.06.035
Shakeel S, Rehman MU, Tabassum N et al (2017) Effect of naringenin (A naturally occurring flavanone) against pilocarpine-induced status epilepticus and oxidative stress in mice. Pharmacogn Mag 13:S154–S160. https://doi.org/10.4103/0973-1296.203977
Xue Y, Xie N, Cao L, Zhao X, Jiang H, Chi Z (2011) Diazoxide preconditioning against seizure-induced oxidative injury is via the PI3K/Akt pathway in epileptic rat. Neurosci Lett 495:130–134. https://doi.org/10.1016/j.neulet.2011.03.054
Shetty AK (2014) Hippocampal injury-induced cognitive and mood dysfunction, altered neurogenesis, and epilepsy: Can early neural stem cell grafting intervention provide protection? Epilepsy Behav 38:117–124. https://doi.org/10.1016/j.yebeh.2013.12.001
Khalil A, Kovac S, Morris G, Walker MC (2017) Carvacrol after status epilepticus (SE) prevents recurrent SE, early seizures, cell death, and cognitive decline. Epilepsia 58:263–273. https://doi.org/10.1111/epi.13645
Long Q, Upadhya D, Hattiangady B, Kim DK, An SY, Shuai B, Prockop DJ, Shetty AK (2017) Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci 114:E3536–E3545. https://doi.org/10.1073/pnas.1703920114
Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27:1766–1791. https://doi.org/10.1038/sj.jcbfm.9600521
Poppe R, Karbach U, Gambaryan S, Wiesinger H, Lutzenburg M, Kraemer M, Witte OW, Koepsell H (1997) Expression of the Na+-D-glucose cotransporter SGLT1 in neurons. J Neurochem 69:84–94
McDonald TS, Carrasco-Pozo C, Hodson MP, Borges K (2017) Alterations in cytosolic and mitochondrial [U- 13 C]-glucose metabolism in a chronic epilepsy mouse model. Eneuro 4:ENEURO.0341-16.2017. https://doi.org/10.1523/ENEURO.0341-16.2017
Vielhaber S, Von Oertzen JH, Kudin AF et al (2003) Correlation of hippocampal glucose oxidation capacity and interictal FDG-PET in temporal lobe epilepsy. Epilepsia 44:193–199
Nehlig A, Rudolf G, Leroy C, Rigoulot MA, Simpson IA, Vannucci SJ (2006) Pentylenetetrazol-induced status epilepticus up-regulates the expression of glucose transporter mRNAs but not proteins in the immature rat brain. Brain Res 1082:32–42. https://doi.org/10.1016/j.brainres.2006.01.078
Folbergrová J, Ješina P, Kubová H, Druga R, Otáhal J (2016) Status epilepticus in immature rats is associated with oxidative stress and mitochondrial dysfunction. Front Cell Neurosci 10:136. https://doi.org/10.3389/fncel.2016.00136
Smeland OB, Hadera MG, McDonald TS et al (2013) Brain mitochondrial metabolic dysfunction and glutamate level reduction in the pilocarpine model of temporal lobe epilepsy in mice. J Cereb Blood Flow Metab 33:1090–1097. https://doi.org/10.1038/jcbfm.2013.54
Schauwecker PE (2012) The effects of glycemic control on seizures and seizure-induced excitotoxic cell death. BMC Neurosci 13:94. https://doi.org/10.1186/1471-2202-13-94
Xia L, Lei Z, Shi Z, Guo D, Su H, Ruan Y, Xu ZC (2016) Enhanced autophagy signaling in diabetic rats with ischemia-induced seizures. Brain Res 1643:18–26. https://doi.org/10.1016/j.brainres.2016.04.054
Chou I-C, Wang C-H, Lin W-D, Tsai FJ, Lin CC, Kao CH (2016) Risk of epilepsy in type 1 diabetes mellitus: a population-based cohort study. Diabetologia 59:1196–1203. https://doi.org/10.1007/s00125-016-3929-0
Maheandiran M, Mylvaganam S, Wu C, el-Hayek Y, Sugumar S, Hazrati L, Campo M, Giacca A et al (2013) Severe hypoglycemia in a juvenile diabetic rat model: presence and severity of seizures are associated with mortality. PLoS One 8:e83168. https://doi.org/10.1371/journal.pone.0083168
Moseley B, Bateman L, Millichap JJ, Wirrell E, Panayiotopoulos CP (2013) Autonomic epileptic seizures, autonomic effects of seizures, and SUDEP. Epilepsy Behav 26:375–385. https://doi.org/10.1016/j.yebeh.2012.08.020
Sabino-Silva R, Mori RC, David-Silva A et al (2010) The Na +-/glucose cotransporters: from genes to therapy. Braz J Med Biol Res 43:1019–1026. https://doi.org/10.1590/S0100-879X2010007500115
Wright EM, Loo DDF, Hirayama BA (2011) Biology of human sodium glucose transporters. Physiol Rev 91:733–794. https://doi.org/10.1152/physrev.00055.2009
Yu AS, Hirayama BA, Timbol G et al (2010) Functional expression of SGLTs in rat brain. Am J Phys Cell Phys 299:C1277–C1284. https://doi.org/10.1152/ajpcell.00296.2010
Yu AS, Hirayama BA, Timbol G et al (2013) Regional distribution of SGLT activity in rat brain in vivo. Am J Phys Cell Phys 304:C240–C247. https://doi.org/10.1152/ajpcell.00317.2012
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th Editio. Academic Press, San Diego
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310. https://doi.org/10.1016/S0076-6879(78)52032-6
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Aebi H (1984) [13] catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Schmued LC, Albertson C, Slikker W (1997) Fluoro-jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res 751:37–46. https://doi.org/10.1016/S0006-8993(96)01387-X
Sanabria ERG, da Silva AV, Spreafico R, Cavalheiro EA (2002) Damage, reorganization, and abnormal neocortical hyperexcitability in the pilocarpine model of temporal lobe epilepsy. Epilepsia 43(Suppl 5):96–106
Tejada J, Garcia-Cairasco N, Roque AC (2014) Combined role of seizure-induced dendritic morphology alterations and spine loss in newborn granule cells with mossy fiber sprouting on the hyperexcitability of a computer model of the dentate gyrus. PLoS Comput Biol 10:e1003601. https://doi.org/10.1371/journal.pcbi.1003601
Yamada A, Momosaki S, Hosoi R, Abe K, Yamaguchi M, Inoue O (2009) Glucose utilization in the brain during acute seizure is a useful biomarker for the evaluation of anticonvulsants: effect of methyl ethyl ketone in lithium-pilocarpine status epilepticus rats. Nucl Med Biol 36:949–954. https://doi.org/10.1016/j.nucmedbio.2009.06.008
Cameron S, Lopez A, Glabman R, Abrams E, Johnson S, Field C, Gulland FMD, Buckmaster PS (2019) Proportional loss of parvalbumin-immunoreactive synaptic boutons and granule cells from the hippocampus of sea lions with temporal lobe epilepsy. J Comp Neurol. https://doi.org/10.1002/cne.24680
Mohapel P, Ekdahl CT, Lindvall O (2004) Status epilepticus severity influences the long-term outcome of neurogenesis in the adult dentate gyrus. Neurobiol Dis 15:196–205. https://doi.org/10.1016/j.nbd.2003.11.010
Meldrum BS, Brierley JB (1973) Prolonged epileptic seizures in primates. Ischemic cell change and its relation to ictal physiological events. Arch Neurol 28:10–17
Meldrum BS, Horton RW (1973) Physiology of status epilepticus in primates. Arch Neurol 28:1–9
Franck G, Sadzot B, Salmon E, Depresseux JC, Grisar T, Peters JM, Guillaume M, Quaglia L et al (1986) Regional cerebral blood flow and metabolic rates in human focal epilepsy and status epilepticus. Adv Neurol 44:935–948
Farooque P, Hirsch L, Levy S, Testa F, Mattson R, Spencer D (2017) Surgical outcome in adolescents with mesial temporal sclerosis: is it different? Epilepsy Behav 69:24–27. https://doi.org/10.1016/j.yebeh.2016.10.028
Fernández-Torre JL, Pascual J, Quirce R, Gutiérrez A, Martínez-Martínez M, Rebollo M (2006) Permanent dysphasia after status epilepticus: long-term follow-up in an elderly patient. Epilepsy Behav 8:677–680. https://doi.org/10.1016/j.yebeh.2006.01.014
Kim HY, Kim JY, Un KG et al (2012) Alien hand syndrome after epilepsia partialis continua: FDG PET and MRI studies. Epilepsy Behav 23:71–73. https://doi.org/10.1016/j.yebeh.2011.08.043
Sakakibara E, Takahashi Y, Murata Y et al (2014) Chronic periodic lateralised epileptic discharges and anti-N-methyl-D-aspartate receptor antibodies. 16:218–222. https://doi.org/10.1684/epd.2014.0655
Van Bogaert P, Goldman S, Rodesch G et al (1994) Cerebral lesions following convulsive partial status epilepticus. Clinical, neuroradiologic and PET study of a case. J Neuroradiol 21:176–180
García-García L, Shiha AA, Fernández de la Rosa R, Delgado M, Silván Á, Bascuñana P, Bankstahl JP, Gomez F et al (2017) Metyrapone prevents brain damage induced by status epilepticus in the rat lithium-pilocarpine model. Neuropharmacology 123:261–273. https://doi.org/10.1016/j.neuropharm.2017.05.007
Sapolsky RM, Stein BA (1989) Status epilepticus-induced hippocampal damage is modulated by glucose availability. Neurosci Lett 97:157–162
Meldrum BS (1983) Metabolic factors during prolonged seizures and their relation to nerve cell death. Adv Neurol 34:261–275
Johansen FF, Diemer NH (1986) Influence of the plasma glucose level on brain damage after systemic kainic acid injection in the rat. Acta Neuropathol 71:46–54
Grimes L, McGinty J, McLain P, Mitchell C, Tilson H, Hong J (1988) Dentate granule cells are essential for kainic acid-induced wet dog shakes but not for seizures. J Neurosci 8:256–264
Lothman EW, Collins RC (1981) Kainic acid induced limbic seizures: metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res 218:299–318. https://doi.org/10.1016/0006-8993(81)91308-1
Shin E-J, Jeong JH, Chung YH, Kim TW, Shin CY, Kim WK, Ko KH, Kim HC (2009) Decrease in the kainate-induced wet dog shake behavior in genetically epilepsy-prone rats: possible involvement of an impaired synaptic transmission to the 5-HT(2A) receptor. J Pharmacol Sci 110:401–404
Lee PH, Hong JS (1990) Ventral hippocampal dentate granule cell lesions enhance motor seizures but reduce wet dog shakes induced by mu opioid receptor agonist. Neuroscience 35:71–77. https://doi.org/10.1016/0306-4522(90)90121-J
Citraro R, Scicchitano F, De Fazio S et al (2011) Preclinical activity profile of α-lactoalbumin, a whey protein rich in tryptophan, in rodent models of seizures and epilepsy. Epilepsy Res 95:60–69. https://doi.org/10.1016/j.eplepsyres.2011.02.013
Clinckers R, Smolders I, Meurs A, Ebinger G, Michotte Y (2004) Anticonvulsant action of hippocampal dopamine and serotonin is independently mediated by D2 and 5-HT1A receptors. J Neurochem 89:834–843. https://doi.org/10.1111/j.1471-4159.2004.02355.x
Kim T-Y, Yi J-S, Chung S-J, Kim DK, Byun HR, Lee JY, Koh JY (2007) Pyruvate protects against kainate-induced epileptic brain damage in rats. Exp Neurol 208:159–167. https://doi.org/10.1016/j.expneurol.2007.08.013
Yang H, Guo R, Wu J, Peng Y, Xie D, Zheng W, Huang X, Liu D et al (2013) The antiepileptic effect of the glycolytic inhibitor 2-deoxy-d-glucose is mediated by upregulation of KATP channel subunits Kir6.1 and Kir6.2. Neurochem Res 38:677–685. https://doi.org/10.1007/s11064-012-0958-z
Stafstrom CE, Ockuly JC, Murphree L, Valley MT, Roopra A, Sutula TP (2009) Anticonvulsant and antiepileptic actions of 2-deoxy-D-glucose in epilepsy models. Ann Neurol 65:435–447. https://doi.org/10.1002/ana.21603
Gasior M, Yankura J, Hartman AL, French A, Rogawski MA (2010) Anticonvulsant and proconvulsant actions of 2-deoxy-d-glucose. Epilepsia 51:1385–1394. https://doi.org/10.1111/j.1528-1167.2010.02593.x
Lian X-Y, Khan FA, Stringer JL (2007) Fructose-1,6-bisphosphate has anticonvulsant activity in models of acute seizures in adult rats. J Neurosci 27:12007–12011. https://doi.org/10.1523/JNEUROSCI.3163-07.2007
Stafstrom CE, Roopra A, Sutula TP (2008) Seizure suppression via glycolysis inhibition with 2-deoxy-D-glucose (2DG). Epilepsia 49:97–100. https://doi.org/10.1111/j.1528-1167.2008.01848.x
Bissonnette P, Gagne H, Blais A, Berteloot A (1996) 2-Deoxyglucose transport and metabolism in Caco-2 cells. Am J Physiol Liver Physiol 270:G153–G162. https://doi.org/10.1152/ajpgi.1996.270.1.G153
Sols A, Crane RK (1954) Substrate specificity of brain hexokinase. J Biol Chem 210:581–595
Kimmich GA, Randles J (1976) 2-Deoxyglucose transport by intestinal epithelial cells isolated from the chick. J Membr Biol 27:363–379
Chen W, Guéron M (1992) The inhibition of bovine heart hexokinase by 2-deoxy-D-glucose-6-phosphate: characterization by 31P NMR and metabolic implications. Biochimie 74:867–873
Yamada K, Ji JJ, Yuan H et al (2001) Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science (80- ) 292:1543–1546. https://doi.org/10.1126/science.1059829
Hernandez-Sanchez C, Basile AS, Fedorova I, Arima H, Stannard B, Fernandez AM, Ito Y, LeRoith D (2001) Mice transgenically overexpressing sulfonylurea receptor 1 in forebrain resist seizure induction and excitotoxic neuron death. Proc Natl Acad Sci 98:3549–3554. https://doi.org/10.1073/pnas.051012898
Ma W, Berg J, Yellen G (2007) Ketogenic diet metabolites reduce firing in central neurons by opening KATP channels. J Neurosci 27:3618–3625. https://doi.org/10.1523/JNEUROSCI.0132-07.2007
Falip M, Miró J, Carreño M, Jaraba S, Becerra JL, Cayuela N, Perez Maraver M, Graus F (2014) Hypoglycemic seizures and epilepsy in type I diabetes mellitus. J Neurol Sci 346:307–309. https://doi.org/10.1016/j.jns.2014.08.024
Huang CW, Cheng JT, Tsai JJ, Wu SN, Huang CC (2009) Diabetic hyperglycemia aggravates seizures and status epilepticus-induced hippocampal damage. Neurotox Res 15:71–81. https://doi.org/10.1007/s12640-009-9008-2
Stafstrom CE (2003) Hyperglycemia lowers seizure threshold. Epilepsy Curr 3:148–149. https://doi.org/10.1046/j.1535-7597.2003.03415.x
de Melo IS, Pacheco ALD, dos Santos YMO, Figueiredo LM, Nicacio DCSP, Cardoso-Sousa L, Duzzioni M, Gitaí DLG et al (2020) Modulation of glucose availability and effects of hypo- and hyperglycemia on status epilepticus: what we do not know yet? Mol Neurobiol:1–15. https://doi.org/10.1007/s12035-020-02133-8
Zamanian G, Shayan M, Rahimi N, Bahremand T, Shafaroodi H, Ejtemaei-Mehr S, Aghaei I, Dehpour AR (2020) Interaction of morphine tolerance with pentylenetetrazole-induced seizure threshold in mice: the role of NMDA-receptor/NO pathway. Epilepsy Behav 112:112. https://doi.org/10.1016/j.yebeh.2020.107343
Eslami F, Rahimi N, Ostovaneh A et al (2020) Sumatriptan reduces severity of status epilepticus induced by lithium–pilocarpine through nitrergic transmission and 5-HT 1B/D receptors in rats: a pharmacological-based evidence. Fundam Clin Pharmacol:fcp.12590. https://doi.org/10.1111/fcp.12590
Taiwe GS, Kouamou ALN, Ambassa ARM, Menanga JR, Tchoya TB, Dzeufiet PDD (2017) Evidence for the involvement of the GABA-ergic pathway in the anticonvulsant activity of the roots bark aqueous extract of Anthocleista djalonensis A. Chev. (Loganiaceae). J Basic Clin Physiol Pharmacol 28:425–435. https://doi.org/10.1515/jbcpp-2017-0048
Vishnoi S, Raisuddin S, Parvez S (2016) Glutamate excitotoxicity and oxidative stress in epilepsy: modulatory role of melatonin. J Environ Pathol Toxicol Oncol 35:365–374. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2016016399
During MJ, Spencer DD (1993) Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet (London, England) 341:1607–1610
Ambrogini P, Torquato P, Bartolini D, Albertini MC, Lattanzi D, di Palma M, Marinelli R, Betti M et al (2019) Excitotoxicity, neuroinflammation and oxidant stress as molecular bases of epileptogenesis and epilepsy-derived neurodegeneration: the role of vitamin E. Biochim Biophys Acta Mol basis Dis 1865:1098–1112. https://doi.org/10.1016/j.bbadis.2019.01.026
Coulter DA, Eid T (2012) Astrocytic regulation of glutamate homeostasis in epilepsy. Glia 60:1215–1226. https://doi.org/10.1002/glia.22341
Rossi AR, Angelo MF, Villarreal A, Lukin J, Ramos AJ (2013) Gabapentin administration reduces reactive gliosis and neurodegeneration after pilocarpine-induced status epilepticus. PLoS One 8:e78516. https://doi.org/10.1371/journal.pone.0078516
Chiu KM, Wu CC, Wang MJ, Lee MY, Wang SJ (2015) Protective effects of bupivacaine against kainic acid-induced seizure and neuronal cell death in the rat hippocampus. Biol Pharm Bull 38:522–530. https://doi.org/10.1248/bpb.b14-00633
Wang LY, Dudek EM, Browning MD, MacDonald JF (1994) Modulation of AMPA/kainate receptors in cultured murine hippocampal neurones by protein kinase C. J Physiol 475:431–437. https://doi.org/10.1113/jphysiol.1994.sp020083
Mishra V, Shuai B, Kodali M, Shetty GA, Hattiangady B, Rao X, Shetty AK (2015) Resveratrol treatment after status epilepticus restrains neurodegeneration and abnormal neurogenesis with suppression of oxidative stress and inflammation. Sci Rep 5. https://doi.org/10.1038/srep17807
Dariani S, Baluchnejadmojarad T, Roghani M (2013) Thymoquinone attenuates astrogliosis, neurodegeneration, mossy fiber sprouting, and oxidative stress in a model of temporal lobe epilepsy. J Mol Neurosci 51:679–686. https://doi.org/10.1007/s12031-013-0043-3
Pestana RRF, Kinjo ER, Hernandes MS, Britto LRG (2010) Reactive oxygen species generated by NADPH oxidase are involved in neurodegeneration in the pilocarpine model of temporal lobe epilepsy. Neurosci Lett 484:187–191. https://doi.org/10.1016/j.neulet.2010.08.049
Yi J-S, Lee S-K, Sato T-A, Koh J-Y (2003) Co-induction of p75(NTR) and the associated death executor NADE in degenerating hippocampal neurons after kainate-induced seizures in the rat. Neurosci Lett 347:126–130
Lee JY, Cole TB, Palmiter RD, Koh JY (2000) Accumulation of zinc in degenerating hippocampal neurons of ZnT3-null mice after seizures: evidence against synaptic vesicle origin. J Neurosci 20:RC79
Lee J-Y, Kim J-H, Palmiter RD, Koh J-Y (2003) Zinc released from metallothionein-iii may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp Neurol 184:337–347
Kim EY, Koh JY, Kim YH, Sohn S, Joe E, Gwag BJ (1999) Zn2+ entry produces oxidative neuronal necrosis in cortical cell cultures. Eur J Neurosci 11:327–334
Kim YH, Kim EY, Gwag BJ, Sohn S, Koh JY (1999) Zinc-induced cortical neuronal death with features of apoptosis and necrosis: mediation by free radicals. Neuroscience 89:175–182
Jiang D, Sullivan PG, Sensi SL, Steward O, Weiss JH (2001) Zn 2+ induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J Biol Chem 276:47524–47529. https://doi.org/10.1074/jbc.M108834200
Sensi SL, Yin HZ, Weiss JH (2000) AMPA/kainate receptor-triggered Zn2+ entry into cortical neurons induces mitochondrial Zn2+ uptake and persistent mitochondrial dysfunction. Eur J Neurosci 12:3813–3818
Kim Y-H, Koh J-Y (2002) The role of NADPH oxidase and neuronal nitric oxide synthase in zinc-induced poly(ADP-ribose) polymerase activation and cell death in cortical culture. Exp Neurol 177:407–418
Noh KM, Koh JY (2000) Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci 20:RC111
Sakurai M, Kurokawa H, Shimada A, Nakamura K, Miyata H, Morita T (2015) Excitatory amino acid transporter 2 downregulation correlates with thalamic neuronal death following kainic acid-induced status epilepticus in rat. Neuropathology 35:1–9. https://doi.org/10.1111/neup.12141
Young CB, Sonne J (2019) Neuroanatomy. StatPearls Publishing, Basal Ganglia
Bosch-Bouju C, Hyland BI, Parr-Brownlie LC (2013) Motor thalamus integration of cortical, cerebellar and basal ganglia information: implications for normal and parkinsonian conditions. Front Comput Neurosci 7:163. https://doi.org/10.3389/fncom.2013.00163
Iseki K, Hanakawa T (2010) The functional significance of the basal ganglia-thalamo-cortical loop in gait control in humans: a neuroimaging approach. Brain Nerve 62:1157–1164
Hooks BM (2017) Sensorimotor convergence in circuitry of the motor cortex. Neurosci 23:251–263. https://doi.org/10.1177/1073858416645088
Ma DL, Qu JQ, Goh ELK, Tang FR (2016) Reorganization of basolateral amygdala-subiculum circuitry in mouse epilepsy model. Front Neuroanat 9:167. https://doi.org/10.3389/fnana.2015.00167
Gröticke I, Hoffmann K, Löscher W (2007) Behavioral alterations in the pilocarpine model of temporal lobe epilepsy in mice. Exp Neurol 207:329–349. https://doi.org/10.1016/j.expneurol.2007.06.021
Lenck-Santini P-P, Holmes GL (2008) Altered phase precession and compression of temporal sequences by place cells in epileptic rats. J Neurosci 28:5053–5062. https://doi.org/10.1523/JNEUROSCI.5024-07.2008
Peixoto-Santos JE, Velasco TR, Galvis-Alonso OY, Araujo D, Kandratavicius L, Assirati JA, Carlotti CG, Scandiuzzi RC et al (2015) Temporal lobe epilepsy patients with severe hippocampal neuron loss but normal hippocampal volume: Extracellular matrix molecules are important for the maintenance of hippocampal volume. Epilepsia 56:1562–1570. https://doi.org/10.1111/epi.13082
Pearson JN, Rowley S, Liang LP, White AM, Day BJ, Patel M (2015) Reactive oxygen species mediate cognitive deficits in experimental temporal lobe epilepsy. Neurobiol Dis 82:289–297. https://doi.org/10.1016/j.nbd.2015.07.005
Folbergrová J, Ješina P, Kubová H, Otáhal J (2018) Effect of resveratrol on oxidative stress and mitochondrial dysfunction in immature brain during epileptogenesis. Mol Neurobiol 55:7512–7522. https://doi.org/10.1007/s12035-018-0924-0
Gao J, Yao H, Pan XD, Xie AM, Zhang L, Song JH, Ma AJ, Liu ZC (2014) Alteration of mitochondrial function and ultrastructure in the hippocampus of pilocarpine-treated rat. Epilepsy Res 108:162–170. https://doi.org/10.1016/j.eplepsyres.2013.11.016
Ren X, Zhou L, Terwilliger R et al (2009) Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci 3:12. https://doi.org/10.3389/neuro.07.012.2009
Acknowledgments
DLGG was supported by the Research Productivity Scholarship Program in Brazilian National Council for Scientific and Technological Development (CNPq). We thank CAPES-Brazil for PhD Research Fellowship to I.S.M., Y.M.O.S., A.L.D.P., M.A.C., and J.F.S.
Funding
This project was supported by FAPEAL, CNPq, and CAPES.
Author information
Authors and Affiliations
Contributions
Conceptualization, I.S.M., R.S.S., and O.W.C.; methodology, I.S.M., Y.M.O.S., A.L.D.P., M.A.C., V.O.S., J.F.S., C.M.B.C., R.C.S.F, A.C.R.L., R.S.S., and O.W.C.; investigation, I.S.M., A.C.R.L., A.U.B., R.S.S., and O.W.C.; formal analysis, I.S.M., R.C.S.F, A.C.R.L., R.S.S., and O.W.C.; supervision and fund acquisition, O.W.C.; writing—review and editing, I.S.M., D.G.L.G., M.D., A.U.B., R.S.S., and O.W.C.; resources, M.D., A.U.B., R.S.S., and O.W.C.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Ethics Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Experiments were performed in accordance with the NIH guidelines for the care and use of laboratory animals, and with approval of the Federal University of Alagoas Animal Use Ethics Committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Online Resource 1
Effects of increased glucose availability on status of oxidative stress levels in rat hippocampus after 24 h of intrahippocampal pilocarpine-induced SE. Modulated levels of malondialdehyde (MDA), total thiol, catalase (CAT), and superoxide dismutase (SOD) were assessed (A). Glucose-(3 mM)-treated rats showed a significant increase in MDA levels (B) and a significant decrease in total thiol (C), CAT (D) and SOD (E) levels compared with saline-treated rats. Error bars indicate the SEM. Data represent the mean ± S.E.M. of 4–6 rats. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with VEH; one-way ANOVA with Dunnett’s post-hoc test. VEH, saline-treated vehicle; H-PILO, pilocarpine and saline; P+G, pilocarpine followed by glucose infusion; DZP, diazepam; SE, Status epilepticus; SEM, standard error of the mean. (PNG 1620 kb)
Online Resource 2
Glucose modulation decreases the cFOS expression in cortical and subiculum areas after intrahippocampal pilocarpine-induced SE. The nuclei were labeled with DAPI (blue, middle panels, A-H2). Fluorescent labeling strong cFOS immunoreactivity (red, left panels) in RSGr (A1), Prh (C1), Pir (E1) and DS (G1) in H-PILO rats. Merge of cFOS and DAPI are shown in right panels (A-H3). Quantitative analysis of cFOS+ neurons in H-PILO (black bars), G+P (gray, blue and red bar) and P+G (gray, blue and red bar outline) rats are shown in B4, D4, F4 and H4. Some concentrations of hippocampal glucose (1, 2 or 3 mM) before and after intrahippocampal pilocarpine attenuated the number of cFOS+ neurons in RSGr (one-way ANOVA, F (6, 22) = 3.59, P = 0.012; B4), Prh (t-test, t5 = 3.79, P = 0.013; D4), Pir (one-way ANOVA, F (6, 16) = 3.70, P = 0.017; F4) and DS (one-way ANOVA, F (6, 20) = 6.51, P = 0.0006; H4) areas. The 3 mM glucose concentration was used as a representative image. Arrows represent the brain areas. Magnification, 200x; scale bar, 50 μm. Error bars indicate the SEM. Data represent the mean ± S.E.M. of 3–6 rats. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with H-PILO; one-way ANOVA with Dunnett’s post-hoc test and unpaired t-test. H-PILO, pilocarpine and saline; G+P, glucose followed by pilocarpine infusion; P+G, pilocarpine followed by glucose infusion; RSGr, retrosplenial; PRh, perirhinal; Pir, piriform; DS, subiculum; SEM, standard error of the mean. (PNG 4306 kb)
Online Resource 3
Increased glucose supply decreases the cFOS expression in thalamic and amygdaloid areas after intrahippocampal pilocarpine-induced SE. The nuclei were labeled with DAPI (blue, middle panels). Fluorescent labeling of the hippocampus shows strong cFOS immunoreactivity (red, left panels) in LPMR (A1), CL (C1), PVP (E1) and LaDL (G1) in H-PILO rats. Merge of cFOS and DAPI shown in right panels. Quantitative analysis of cFOS+ neurons in H-PILO (black bars), G+P (gray, blue and red bar) and P+G (gray, blue and red bar outline) rats are shown in B4, D4, F4 and H4. Some concentrations of hippocampal glucose (1, 2 or 3 mM) before and after intrahippocampal pilocarpine decreased the number of cFOS+ neurons in LPMR (one-way ANOVA, F (6, 22) = 10.94, P < 0.0001; B4), CL (one-way ANOVA, F (6, 22) = 3.54, P = 0.01; D4), PVP (one-way ANOVA, F (6, 20) = 5.21, P = 0.0023; F4) and LaDL (one-way ANOVA, F (3, 9) = 9.65, P = 0.0036; H4) areas. The 3 mM glucose concentration was used as a representative image. Arrows represent the brain areas. Magnification, 200x; scale bar, 50 μm. Error bars indicate the SEM. Data represent the mean ± S.E.M. of 5–6 rats. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 compared with H-PILO; one-way ANOVA with Dunnett’s post-hoc test. H-PILO, pilocarpine and saline; G+P, glucose followed by pilocarpine infusion; P+G, pilocarpine followed by glucose infusion; PVP, posterior paraventricular th ncl; CL, centrolateral th ncl; LPMR, lateral posterior th ncl; LaDL, lateral amygdaloid ncl; SEM, standard error of the mean. (PNG 4591 kb)
Online Resource 4
Glucose supply reduces the seizures severity but does not change memory dysfunction and oxidative stress. Cell death and neuronal activity are attenuated in the hippocampus and extrahippocampal areas because of this glucose control, via elevation of sodium-glucose cotransporter translocation in hippocampus. (PNG 3670 kb)
Rights and permissions
About this article
Cite this article
de Melo, I.S., dos Santos, Y.M.O., Pacheco, A.L.D. et al. Role of Modulation of Hippocampal Glucose Following Pilocarpine-Induced Status Epilepticus. Mol Neurobiol 58, 1217–1236 (2021). https://doi.org/10.1007/s12035-020-02173-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02173-0