Abstract
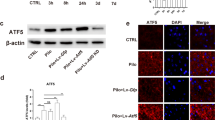
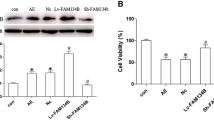
The mitochondrial unfolded protein response (mtUPR)-a stress response pathway for maintaining protein homeostasis-is critical in seizures-induced neuronal injury. The activating transcription factor 5 (ATF5) regulates mtUPR; however, whether ATF5-regulated mtUPR has a role in neuronal injury in epilepsy remains uncertain. Here, we investigated the effects of ATF5-regulated mtUPR on neuronal injury in hippocampal neurons with seizures evoked by Mg2+-free medium. HSP60 and ClpP, key proteins of mtUPR, were upregulated, indicating mtUPR activation. ATF5 overexpression by lentiviral vector infection potentiated mtUPR, whereas ATF5 downregulation by lentiviral vector infection attenuated this response. Moreover, ATF5 overexpression elevated mitochondrial membrane potential and reduced reactive oxygen species (ROS) generation, suggesting that ATF5 overexpression protected mitochondrial homeostasis, while ATF5 downregulation had the opposite effect. ATF5 overexpression also reversed Bcl2 downregulation and Bax upregulation and attenuated seizures-induced neuronal apoptosis, while ATF5 downregulation aggravated the injury. Our study demonstrates that ATF5 attenuates seizures-induced neuronal injury, possibly by regulating mtUPR pathways, to prevent mitochondrial dysfunction.







Similar content being viewed by others
Data Availability
All data generated by the current study are available upon reasonable request.
Code Availability
Not applicable.
References
Singh S, Singh TG, Rehni AK, Sharma V, Singh M, Kaur R (2021) Reviving mitochondrial bioenergetics: a relevant approach in epilepsy. Mitochondrion 58:213–226. https://doi.org/10.1016/j.mito.2021.03.009
Munch C (2018) The different axes of the mammalian mitochondrial unfolded protein response. BMC Biol 16:1–9. https://doi.org/10.1186/s12915-018-0548-x
Zhou H, Ren J, Toan S, Mui D (2021) Role of mitochondrial quality surveillance in myocardial infarction: from bench to bedside. Ageing Res Rev 66:101250. https://doi.org/10.1016/j.arr.2020.101250
Ozkurede U, Miller RA (2019) Improved mitochondrial stress response in long-lived Snell dwarf mice. Aging Cell 18(6):e13030. https://doi.org/10.1111/acel.13030
Duncan OF, Granat L, Ranganathan R, Singh VK, Mazaud D, Fanto M, Chambers D, Ballard CG, Bateman JM (2018) Ras-ERK-ETS inhibition alleviates neuronal mitochondrial dysfunction by reprogramming mitochondrial retrograde signaling. PLoS Genet 14(7):1007567. https://doi.org/10.1371/journal.pgen.1007567
Suomalainen A, Battersby BJ (2018) Mitochondrial diseases: the contribution of organelle stress responses to pathology. Nat Rev Mol Cell Biol 19(2):77–92. https://doi.org/10.1038/nrm.2017.66
Kim J-E, Park H, Kim T-H, Kang T-C (2021) LONP1 Regulates mitochondrial accumulations of HMGB1 and caspase-3 in CA1 and PV neurons following status epilepticus. Int J Mol Sci 22(5):2275. https://doi.org/10.3390/ijms22052275
O’Malley J, Kumar R, Inigo J, Yadava N, Chandra D (2020) Mitochondrial stress response and cancer. Trends Cancer 6(8):688–701. https://doi.org/10.1016/j.trecan.2020.04.009
Deng P, Haynes CM (2017) Mitochondrial dysfunction in cancer: potential roles of ATF5 and the mitochondrial UPR. Semin Cancer Biol 47:43–49. https://doi.org/10.1016/j.semcancer.2017.05.002
Bordt EA, Smith CJ, Demarest TG, Bilbo SD, Kingsbury MA (2019) Mitochondria, oxytocin, and vasopressin: unfolding the inflammatory protein response. Neurotox Res 36(2):239–256. https://doi.org/10.1007/s12640-018-9962-7
Melber A, Haynes CM (2018) UPRmt regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res 28(3):281–295. https://doi.org/10.1038/cr.2018.16
Ryoo HD, Vasudevan D (2017) Two distinct nodes of translational inhibition in the Integrated Stress Response. BMB Rep 50(11):539–545. https://doi.org/10.5483/BMBRep.2017.50.11.157
Abele R, Tampe R (2018) Moving the cellular peptidome by transporters. Front Cell Dev Biol 6:43. https://doi.org/10.3389/fcell.2018.00043
Gao X, Jiang Z, Yan X, Liu J, Li F, Liu P, Li J, Wei Y, Sun YE, Zhang Y, Wang C (2021) ATF5, a putative therapeutic target for the mitochondrial DNA 3243A > G mutation-related disease. Cell Death Dis 12(7):1–11. https://doi.org/10.1038/s41419-021-03993-1
Wang YT, Lim Y, McCall MN, Huang KT, Haynes CM, Nehrke K, Brookes PS (2019) Cardioprotection by the mitochondrial unfolded protein response requires ATF5. Am J Physiol-Heart Circ Physiol 317(2):H472–H478. https://doi.org/10.1152/ajpheart.00244.2019
Feng S, Ma S, Jia C, Su Y, Yang S, Zhou K, Liu Y, Cheng J, Lu D, Fan L, Wang Y (2016) Sonic hedgehog is a regulator of extracellular glutamate levels and epilepsy. EMBO Rep 17(5):682–694. https://doi.org/10.15252/embr.201541569
Xu B, Qin Y, Li D, Cai N, Wu J, Jiang L, Jie L, Zhou Z, Xu J, Wang H (2020) Inhibition of PDE4 protects neurons against oxygen-glucose deprivation-induced endoplasmic reticulum stress through activation of the Nrf-2/HO-1 pathway. Redox Biol 28:101342. https://doi.org/10.1016/j.redox.2019.101342
Wang C, Li Y, Li Y, Du L, Zhang J, Li N, Hu X, Zhang W, Xie N, Ming L (2021) FAM134B-mediated ER-Phagy in Mg2+-free solution-induced mitochondrial calcium homeostasis and cell death in epileptic hippocampal neurons. Neurochem Res 46(9):2485–2494. https://doi.org/10.1007/s11064-021-03389-9
Sombati S, Delorenzo RJ (1995) Recurrent spontaneous seizure activity in hippocampal neuronal networks in culture. J Neurophysiol 73(4):1706–1711. https://doi.org/10.1152/jn.1995.73.4.1706
Singh S, Singh TG (2021) Emerging perspectives on mitochondrial dysfunctioning and inflammation in epileptogenesis. Inflamm Res 70(10–12):1027–1042. https://doi.org/10.1007/s00011-021-01511-9
Paleologou E, Ismayilova N, Kinali M (2017) Use of the ketogenic diet to treat intractable epilepsy in mitochondrial disorders. J Clin Med 6(6):56. https://doi.org/10.3390/jcm6060056
Mahmud SA, Qureshi MA, Sapkota M, Pellegrino MW (2020) A pathogen branched-chain amino acid catabolic pathway subverts host survival by impairing energy metabolism and the mitochondrial UPR. PLoS Pathog 16(9):e1008918. https://doi.org/10.1371/journal.ppat.1008918
Smyrnias I (2021) The mitochondrial unfolded protein response and its diverse roles in cellular stress. Int J Biochem Cell Biol 133:105934. https://doi.org/10.1016/j.biocel.2021.105934
Paz JL, Levy D, Oliveira BA, de Melo TC, de Freitas FA, Reichert CO, Rodrigues A, Pereira J, Bydlowski SP (2019) 7-Ketocholesterol promotes oxiapoptophagy in bone marrow mesenchymal stem cell from patients with acute myeloid leukemia. Cells 8(5):482. https://doi.org/10.3390/cells8050482
Zhang Y, Wang Y, Xu JN, Tian F, Hu SY, Chen YD, Fu ZH (2019) Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J Pineal Res 66(2):e12542. https://doi.org/10.1111/jpi.12542
Wu LQ, Xiong XX, Wu XM, Ye YZ, Jian ZH, Zhi Z, Gu LJ (2020) Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Front Mol Neurosci 13:28. https://doi.org/10.3389/fnmol.2020.00028
Wanderoy S, Hees JT, Klesse R, Edlich F, Harbauer AB (2021) Kill one or kill the many: interplay between mitophagy and apoptosis. Biol Chem 402(1):73–88. https://doi.org/10.1515/hsz-2020-0231
Yilmaz EN, Bay S, Ozturk G, Ucisik MH (2020) Neuroprotective effects of curcumin-loaded emulsomes in a laser axotomy-induced CNS injury model. Int J Nanomed 15:9211–9229. https://doi.org/10.2147/ijn.S272931
Zhou H, Toan S, Zhu P, Wang J, Ren J, Zhang Y (2020) DNA-PKcs promotes cardiac ischemia reperfusion injury through mitigating BI-1-governed mitochondrial homeostasis. Basic Res Cardiol 115(2):1–17. https://doi.org/10.1007/s00395-019-0773-7
Acknowledgments
We acknowledge the support of the Translational Medicine Platform of Academy of Medical Sciences, Zhengzhou University.
Funding
This work was supported by the National Natural Science Foundation of China (81971214, 81701272), and Provincial Ministry Co-construction Project from Medical Scientific and Technological Research Program of Henan Province (SB201902011).
Author information
Authors and Affiliations
Contributions
XYW, XMY, and LYD performed the experiments. XYW, YJL, and FXL analyzed the data. The first draft of the manuscript was written by XYW. CW and NCX contributed to the study conception and design, and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors claim that there are no conflicts of interest.
Ethical Approval
The study was approved by the ethics committee of Zhengzhou University, and the experiment was conducted in strict accordance with international standards.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Yu, X., Li, Y. et al. ATF5 Attenuates Apoptosis in Hippocampal Neurons with Seizures Evoked by Mg2+-Free Medium via Regulating Mitochondrial Unfolded Protein Response. Neurochem Res 48, 62–71 (2023). https://doi.org/10.1007/s11064-022-03702-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03702-0