Abstract
The mechanisms underlying cerebral vascular dysfunction and edema during hepatic encephalopathy (HE) are unclear. Blood–brain barrier (BBB) impairment, resulting from increased vascular permeability, has been reported in acute and chronic HE. Mitochondrial dysfunction is a well-documented result of HE mainly affecting astrocytes, but much less so in the BBB-forming endothelial cells. Here we review literature reports and own experimental data obtained in HE models emphasizing alterations in mitochondrial dynamics and function as a possible contributor to the status of brain endothelial cell mitochondria in HE. Own studies on the expression of the mitochondrial fusion-fission controlling genes rendered HE animal model-dependent effects: increase of mitochondrial fusion controlling genes opa1, mfn1 in cerebral vessels in ammonium acetate-induced hyperammonemia, but a decrease of the two former genes and increase of fis1 in vessels in thioacetamide-induced HE. In endothelial cell line (RBE4) after 24 h ammonia and/or TNFα treatment, conditions mimicking crucial aspects of HE in vivo, we observed altered expression of mitochondrial fission/fusion genes: a decrease of opa1, mfn1, and, increase of the fission related fis1 gene. The effect in vitro was paralleled by the generation of reactive oxygen species, decreased total antioxidant capacity, decreased mitochondrial membrane potential, as well as increased permeability of RBE4 cell monolayer to fluorescein isothiocyanate dextran. Electron microscopy documented enlarged mitochondria in the brain endothelial cells of rats in both in vivo models. Collectively, the here observed alterations of cerebral endothelial mitochondria are indicative of their fission, and decreased potential of endothelial mitochondria are likely to contribute to BBB dysfunction in HE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatic Encephalopathy: A Brief Overview of Clinical Characteristics and Pathogenesis
Hepatic encephalopathy (HE) is a complex neuropsychiatric disorder that results from impaired liver function. As a consequence, insufficient clearance of toxins from blood, mainly ammonia, results in their accumulation in the brain. Impaired liver function, associated with acute, chronic liver failure or cirrhosis, often results in a wide range of neurological alterations, including cognitive and motor disturbances [1].
The cellular and molecular mechanisms underlying HE are complex and have not yet been fully deciphered. However, there is a consensus that ammonia, as a major neurotoxin, interferes with various aspects of brain metabolism and neural transmission, and impairs cerebral water-ion homeostasis [2, 3]. The above-listed abnormalities contribute to brain edema, the key pathologic manifestation of acute HE [4, 5]. The important role of inflammation in the pathogenesis of HE is being more and more emphasized [6,7,8].
In acute HE, brain edema leads to the patients’ death in more than 50% of cases, which is a consequence of increased intracranial pressure and herniation [4, 9]. While by analogy to other brain pathologies, brain edema associated with HE is likely elicited by a combination of cytotoxic and vasogenic factors their relative roles have long remained a matter of debate [4, 10].
The well-established view is that in brain astrocytes detoxify ammonia by an enzymatic reaction catalyzed by glutamine synthase [11]. The subsequent accumulation of glutamine most likely results in cellular edema formation, despite potential compensatory mechanisms. In agreement with the long-held view that HE is a primarily gliopathy [11, 12]. The development of brain edema in HE is believed to be primarily due to pathological cell swelling referred as cytotoxic edema. Cytotoxic component of brain edema in HE is thought to primarily reflect swelling of astrocytes by mechanisms related to intracellular metabolic and ion imbalance and the ensuing intracellular accumulation of water [13]. The molecular mechanisms underlying HE-induced cytotoxic edema have been relatively well delineated. Both clinical and animal model studies favor the direct role of ammonia in inducing cytotoxic components of brain edema. The current view proposes that ammonia induced astrocytic swelling by a complex interplay of (i) oxidative/nitrosative stress (ONS) (ii) impairment of import and export of osmotically active substances leading to intracellular osmotic imbalance, (iii) mitochondrial dysfunction related to excessive accumulation of ammonia-derived glutamine and successive intra-mitochondrial release of neurotoxic concentrations of ammonia [12, 14]. Some details warrant to be the highlight here.
In the astrocytic mitochondria, ammonia induces ONS by the formation of free radicals that in turn lead to the pathological condition called mitochondrial permeability transition pore (mPTP) [15]. The mPTP is characterized by a rapid loss of inner membrane potential and collapsed mitochondrial ATP-synthesis. Additionally, high intracellular Ca2+ concentration is considered to mediate mPTP and altered mitochondrial redox-state and pH [16]. Although mPTP has been extensively investigated due to its involvement in apoptosis in different diseases (e.g., traumatic brain injury, neurodegenerative diseases, and ischemia–reperfusion) the pore complex in the mitochondrial inner membrane responsible for the increase in permeability has not been structurally identified [17]. The occurrence of mPTP in ammonia-treated cultured astrocytes has been associated with cell volume increase suggesting its role in astrocyte swelling [18]. Furthermore, cultured astrocytes treated with ammonia and different cytokines: TNF-α, interleukin-1β, interleukin-6, and interferon-γ, presented the induction of the mPTP in a time-dependent and additive manner [19]. Studies on patients with HE have shown metabolic disturbances in the brain indicating a compromised oxidative metabolism most likely due to mitochondrial dysfunction, that were correlated with elevated levels of glutamine [20].
Experimental evidence underscores the contribution of the inflammatory component (peripheral or intracerebral) to cytotoxic brain edema [21, 22]. In turn, the term vasogenic brain edema reflects water accumulation in the extracellular space, and is related to its uncontrolled flux across a selective blood–brain barrier (BBB), in association with peripheral osmotically active substances. In contrast to cytotoxic edema, the role of the vasogenic component in the induction of brain edema in HE has been disputed [23]. The evidence is still contradictory and the underlying mechanisms remain obscure.
Below we discuss BBB alterations in HE pathology, and the discussion is preceded by a characterization of the composition and cytoarchitecture of BBB. Next, we discuss and present data regarding HE-related impairment in processes that contribute to endothelial dysfunction and culminate in increased BBB dysfunctionality, which is understood as a consequence of the disruption in mitochondrial quality control processes.
Blood–Brain Barrier
The histological structure of the BBB is organized by the basement membrane, endothelial cells, pericytes, and astrocyte end-feet. The penetrability of this highly selective fastener between blood and CNS is controlled by two checkpoints: (1) the endothelial cells, whose cohesive properties and physical resistance of, supported by the inter-endothelial tight junction (TJ) proteins: zonula occludens-1 (ZO-1), claudin-5, occludin, junctional adhesion molecules (JAM); and, the adherents complexes, E-cadherin; and (2) the basal lamina. Both the endothelial cell permeability barrier and the basal lamina matrix derive from the cooperation between the endothelium and astrocytes and together constitute the BBB [24]. The endothelial cell barrier properties are only partly attributable to TJs proteins [25]; an important role is played by the stability of the endothelial cell–astrocyte assembly, which requires matrix adhesion. This view is supported by (i) high expression of integrins and dystroglycan at the BBB; (ii) correlation of alterations in the expression of integrins and dystroglycan with the degree of BBB permeability, and subsequent glial activation and neuronal injury; (iii) breakdown of the cerebral vasculature in transgenic mice in which specific integrins are absent [26]. The above considerations have collectively implicated that BBB consists of an array of different components, including TJs and the inter-endothelial adherens complexes (ACs), matrix adhesion complexes formed between adhesion receptors integrins on both endothelial cells and astrocytes, dystroglycan, a single heterodimeric transmembrane receptor, distinct from integrins that forms a physical link between the intracellular cytoskeleton and the extracellular matrix and anchors astrocytes (for reviews and references see del Zoppo and Milner [27]). Of note, vertical adhesion could be a central determinant of the endothelial portion of the intact BBB. This statement is supported by data indicating disturbed BBB permeability and decreased occludin-5 expression in mice after functional blocking of 1β-integrin [28].
Pericytes are important constituents of BBB regulating its functional aspects. While pericytes do not per se induce BBB-specific gene expression in endothelial cells, they inhibit the expression of molecules that increase vascular permeability and CNS immune cell infiltration [29]. Only recently, a link between pericytes’ loss and symptoms associated with neurologic diseases has begun to be elucidated [30, 31]. Uniquely positioned within the neurovascular unit between endothelial cells of brain capillaries, astrocytes, and neurons, pericytes regulate BBB formation and maintenance, vesicle trafficking in endothelial cells, vascular stability, capillary blood flow, and clearance of toxic cellular by-products necessary for normal functioning of the CNS [32]. Whether pericytes’ impairment contributes to BBB dysfunction in HE has not been addressed.
Alteration of the BBB Function and Structure in HE
Fundamental ultrastructural analysis of the cerebral cortex of patients diagnosed with ALF reported by Kato et al., documented swelling of astrocytes end-feet and increased number of vacuoles and vesicles in endothelial cells and pericytes. The study also documented that the basement membrane were enlarged, with generalized rarefaction and vacuolization, while TJs of endothelial cells were intact [33]. The above findings were indicative of BBB disruption in ALF. Otherwise, the contribution of BBB impairment to HE has been a matter of contradictory reports. Rats with severe, acute HE induced by ip. injection of galactosamine and azoxymethane, have presented brain extravasation to Evans Blue and alpha-aminoisobutyric acid, the classical markers of BBB leakage and altered transport, respectively [34, 35]. The reduction in the expression of TJs proteins (occludin, claudin-5, ZO-1, -2) contributing to a reduction in the integrity of the BBB have become evident in brains from this rat models [36, 37]. On the other hand, in galactosamine-induced acute HE in the rabbit, capillary endothelial cells appeared normal, and no evidence of brain extravasation to horseradish peroxidase was observed [38]. Discrepant results have been obtained in animal models of acute HE based on hepatic devascularization [39, 40].
Reported inconsistencies in the observed BBB impairment may be due to differences in the use of animal species and/or acuteness vs chronicity of HE (etiology; toxins/surgical procedures). In a rat model of chronic HE induced by bile duct ligation (BDL), an electron microscopy study revealed anatomically intact BBB structure [41] and decomposition of ZO proteins expression [42]. Furthermore, in the same model, no brain extravasation of Evans blue or sodium fluorescein was found [9], nor any changes in the expression of the TJs proteins were detected. No breakdown of the BBB was likewise demonstrated in a rat model of chronic HE induced by portocaval anastomosis [43]. It is unknown, whether HE may lead to alterations in the matrix elements, matrix adhesion receptor expression by both endothelial cells and astrocytes, factors affecting vascular permeability. Concluding, alternations of the BBB resulting in permeability increase were reported in most of reproducible and well-characterized animal models of HE (Table 1).
In the above mentioned studies chronic HE produced minimal HE and, therefore, it remains to be determined whether BBB breakdown is associated with overt HE. Interestingly, a magnetic resonance imaging study in cirrhotic patients demonstrated the co-existence of both cytotoxic and vasogenic brain edema [64]. Early works indicates a significant increase in the BBB permeability for ammonia [65], but subsequent studies have not substantiated alternations of the permeability-surface area of the BBB for ammonia in HE [66, 67]. Collectively, the role of increased permeability of the BBB in brain edema development and/or progression deserves more complex and detailed investigation (for discussion see Scott et al. [21]).
Importantly, some studies underscore the contribution of inflammatory factors to the vasogenic component of brain edema under HE. Inflammatory mediators in peripheral blood are not able to cross the BBB due to their molecular size. However, different types of cytokines can modulate endothelial TJs and thus unlock the BBB [68], or initiate endothelial inflammatory processes that involve downstream cyclooxygenase (COX), prostanoids, and NO signaling, allowing the direct interaction with astrocytes. Increased brain levels of TNFα, IL-1β, and IL6 and activation of microglia as a source for intracerebral cytokine production were reported in experimental models of ALF [69]. Upon systemic inflammation, microglial cells and astrocytes have been shown to release proinflammatory cytokines, which were suggested to contribute to enhanced neuropsychological impairment induced by hyperammonemia [11, 70, 71]. The study by Lv et al. suggested that deleterious effects of systemic inflammation for the brain are linked with the observed alterations in the BBB [37]. Mentioned study documented the crucial role of TNF-α in the development of BBB abnormalities and corroborated well with the observation of increased TNF-α in patients with ALF [37]. In acetaminophen-induced ALF mice, it was found that increases in BBB permeability positively correlated with elevated serum TNF-α levels, which could be prevented by administering anti-TNFα-IgG [47]. Similarly, TNF-α or TNF-α-R1 antibodies increased the expression of TJs proteins occludin and ZO-1, which result in decreased leakage of Evans Blue in brains of ALF mice induced by d-galactosamine and lipopolysaccharide [72]. Additionally, a possible mutual relation between neuroinflammation, cerebral blood flow, and intracranial hypertension have been suggested [73, 74].
Because cerebral endothelial dysfunction is often associated with compromised BBB, understanding the endothelial factors that regulate vessel function to maintain BBB role and prevent vascular permeability may provide insights into disease prevention and treatment.
Mitochondrial-Derived Reactive Oxygen Species (ROS) in the Endothelial Cells: Implication to Cerebral Dysfunction Observed in HE
Endothelium relies predominantly on anaerobic glycolysis for ATP turnover and mitochondria make up 2–5% of the cytoplasmic volume of endothelial cells in most vascular units [75]. Besides its metabolic role mitochondria integrate signals from the environment, perceive cellular stresses, control cell death signaling to list a key function. Since mitochondria as a major source of oxidative stress contribute to the pathogenesis of HE, its role as a potential trigger of the brain endothelial dysfunction upon HE should be uncovered.
Mitochondrial ROS generation in the endothelial cells is considered as one of the primary cell signaling pathways. Endothelial cells produce different types of ROS, including superoxide (O2−), hydrogen peroxide (H2O2), peroxynitrite (ONOO–), hydroxyl radicals (OH⋅), and other reactive oxygen and nitrogen species [76, 77].
However, oxidative stress that results from increased oxidant production, reduced antioxidant capacity or both, leads to endothelial dysfunction by reducing nitric oxide (NO) bioavailability [78, 79]. Importantly, other sources of ROS in the vessels include NADPH oxidase (NOX) and xanthine oxidase [77].
Ammonia was found to cause the increased generation of ROS in rat brain endothelial cell line (RBE4) [80] and primary cultures of brain ECs [81]. In RBE4 cells, treatment with ammonia increased permeability of endothelial cells monolayer to fluorescein isothiocyanate (FITC)-dextran (40 kDa). This effect was ameliorated by co-treatment with a matrix metalloproteinase inhibitor, or an antioxidant, glutathione diethyl ester [80]. The matrix metalloproteinase 9 (MMP9) was also increased in the brain of BDL rats which accompanied an increase in permeability to sodium fluorescein, Evans blue, and FITC-dextran along with an increase in brain water content [82].
Increased levels of hydroxyl radicals were observed in rat brain vessels isolated from thioacetamide-induced HE [83]. An increase of hydroxyl radicals (OH⋅) was observed in rat brains in vivo after direct infusion of ammonium chloride to the striatum through a microdialysis probe [84]. Increased levels of nitrites and nitrates (markers of NO production) were also frequently detected in the brains of animals with experimentally induced HE [85,86,87]. Additionally, hyperammonemia in vivo was associated with increased expression and activity of heme oxygenase-1 (HO-1), a ubiquitous marker of oxidative stress [88, 89].
It has been shown that exogenously added l-glutamine reduces NO generation in the brain by inhibiting l-arginine transport via y + LAT2 exchanger. This effect was additionally potentiated after a direct infusion of ammonia to the brain via microdialysis probe [87], or when l-glutamine accumulated in there during HE [86]. Tentatively, the mechanism may also operate in the cerebral capillary endothelial cells forming the BBB, where enhanced glutamine level also would modulate l-arginine transport. Of note, increased expression of y + LAT2 was observed in RBE4 cells upon ammonia exposure [80], and in rat brains upon hyperammonemia in situ [86]. However, the outcome of carrier operation would depend on l-glutamine cell membrane gradient. The importance of this mechanism comes to light when confronted with the previous observation that l-glutamine infusion in the absence of hyperammonemia impairs cerebrovascular CO2 reactivity, most likely by reducing l-arginine availability and NO synthesis [90]. A recent study from our laboratory demonstrated strong differences in the reactivity of the middle cerebral arteries and in their response to extravascular l-arginine application between vessels isolated from rats with TAA-induced HE and control animals, implicating that impaired vascular tone of cerebral arteries, which may involve, among other factors, their persistent exposure to high l-glutamine [91].
Excess of ROS reduces NO bioavailability through mechanisms including NO scavenging that occurs when O2− reacts with NO to form ONOO−, which broadly contributes to cellular ONS and uncoupling of endothelial nitric oxide synthase (eNOS) [92]. Moreover, dissociation of eNOS monomers in the uncoupled eNOS is associated with a higher ratio of eNOS monomer-to-eNOS dimers [83, 93]. In addition, O2− can oxidize the essential eNOS cofactor BH4 to BH2, which subsequently leads to eNOS uncoupling whereby eNOS produces more O2− and less NO and lead to inactivation of proteins through the nitration of tyrosine residues. Such proteins include the antioxidant enzyme MnSOD [94, 95].
In our very recent study, a decrease of eNOS content and its uncoupling concurred with and was likely causally related to, both increased brain content of ROS and decreased cerebral cortical blood flow (CBF) in the thioacetamide-induced animal model of acute HE [83].
The specific mechanisms by which mitochondria in the endothelium release ROS and uncouple eNOS involve mitochondrial ATP-dependent potassium channel (MitoK+ATP) activation and subsequent induction of mPTP. MitoK+ATP activity is regulated by falling ATP and rising ADP levels, thus linking cellular metabolism with membrane excitability [96].
It was recently reported, that the MitoK+ATP channel is involved in Parkinson’s disease (PD) mainly via the regulation of mitochondrial biogenesis and fission/fusion [97]. At the molecular level, the authors using in vivo and in vitro rotenone models of PD documented that the pore subunit of Kir6.1, the major component of the MitoK+ATP channel was the key contributor in its interaction with mitochondrial dynamics.
It is important to underline that mitochondria are both the source and a target of excess ROS. Excessive generation of ROS, particularly of ONOO− can result in oxidative damage to the mitochondrial respiratory complexes [98]. Cytoplasmic ROS may elicit mitochondria depolarization, at least in part through the opening of MitoK+ATP channels, which results in mitochondrial ROS release by respiratory complexes and the mPTP. The release of mitochondrial ROS may further activate NADPH oxidase via protein kinase C, resulting in increased cytoplasmic O−2 production and reduced NO bioavailability [99]. On the other hand, hyperammonemia was also found to be associated with a decreased activity of antioxidant enzymes (glutathione peroxidase, superoxide dismutase, and catalase) in the brain, both in the cytosolic and mitochondrial fractions [100]. In line, glutathione accumulation in the extracellular space of rat prefrontal cortex upon ammonia infusion was previously documented [101]. Of note, manipulations resulting in the recovery of the enzyme activities of the GSH metabolism ameliorated HE symptoms both in experimental animals [102] and in human patients [103]. Moreover, excess ONOO− can also lead to inactivation of the endogenous proteins, antioxidant mechanisms (i.e., mitochondrial SOD2) through nitration. Changes in nitrated proteins content supporting this scenario were observed in brain homogenates obtained from rats with TAA-induced acute HE [83]. The above data support the concept that ONS contribute to the alterations in BBB permeability and thus to the vasogenic component of cerebral edema associated with HE.
Aberrant Mitochondrial Quality Control Linked to Endothelial Dysfunction
The role of mitochondria in brain endothelial cells has previously been underestimated since vascular endothelial cells are located in juxtaposition to types of cells, that heavily rely on oxidative phosphorylation. Such cells, which in the periphery are represented by skeletal muscle cells and cardiomyocytes, predominantly rely on anaerobic glycolysis for ATP turnover, and mitochondria make up 2–5% of their cytoplasmic volume [104].
The location of the mitochondria within the endothelial cell of different organs and locations differs, largely depending on the signaling required. For instance, in pulmonary artery endothelial cells where oxygen sensing is relevant, mitochondria are localized near the nucleus, ensuring hypoxia-induced transcriptional regulation [105]. In turn in coronary arterioles, endothelial mitochondria are anchored to the cytoskeleton, initiating vasodilation in response to shear stress [79].
Cellular mitochondrial content is tightly regulated and is determined by the balance between mitochondrial biogenesis and degradation through the process called mitophagy, the form of autophagy in mitochondria (removal of damaged organelles). Mitochondrial organization, which is fundamental in determining their function, is determined by the balance between fusion and fission that determines the mitochondrial number, morphology, and size (Fig. 1) [106]. The cytoskeletal organization, which has a relevant role in maintaining mitochondrial network and function [107]. Mitochondrial quality control is required for optimal mitochondrial function, therefore dysregulation of these processes due to disease-related alterations initiate mitochondria-mediated cell senescence and apoptosis [78, 108]. In the brain, pathological state-associated disturbances in mitochondria quality control purportedly leading to cerebral endothelial dysfunction, and BBB impairment have gained attention only lastly [109]. In more general terms, the imbalance between controlling processes was documented and discussed in various disorders such as cancer, neurodegenerative and cardiovascular diseases [110,111,112].
Schema of fusion/fission processes in the mitochondria. Mitofusin 1/2 and OPA1 are major proteins that control mitochondrial fusion. Mfn1 and Mfn2 locate in the outer mitochondrial membrane with their GTPase site facing the cytosol to coordinate the fusion process with the outer membrane of opposing mitochondria. OPA1 protein, localized in the intermembrane side, controls the fusion of the inner mitochondria membrane. DRP1, Mff, and Fis1 proteins regulated mitochondrial fission. DRP1 is localized in the cytosol and recruited to the outer mitochondrial membrane during fission. Fis1 and Mff are located in the outer mitochondrial membrane and work as the adaptor for DRP1
Decreased mitochondrial biogenesis, one of the deregulatory events in the endothelium, is related to the regulator’s peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and mitochondrial transcription factor A (TFAM) [113]. Further, dysregulation of mitochondrial dynamics is caused by an imbalance between proteins involved in fission [dynamin-related protein 1 (DRP1) and fission 1 (FIS1)] and fusion (transmembrane GTPases mitofusin 1 (MFN1) and 2 (MFN2) and the optic atrophy protein 1 (OPA1)). Mitochondrial fusion allows the transfer of gene products between mitochondria for proper functioning in normal conditions and specifically during metabolic and oxidative stress. In turn, mitochondrial fission is crucial for mitochondrial division and quality control. The role of mitochondrial failure was often described as associated with dysfunctional BBB in different neurologic disorders, including stroke, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and epilepsy [114,115,116,117,118,119]. In so far, the role of altered mitochondrial dynamics involving fission, fusion, and mitophagy processes, has not been adequately explored in the context of BBB dysfunction upon HE.
Effects of HE-Key Factors: Ammonia and TNFα on the Mitochondrial Membrane Potential, the Expression of Genes Involved in Mitochondrial Fusion and Fission, and Morphology of Mitochondria in Cerebral Endothelial Cell
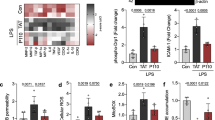
We analyzed mitochondrial membrane potential and the expression of genes involved in mitochondrial fusion and fission in the rat brain endothelial cells (RBE4 cell line) treated with 5 mM ammonium chloride “ammonia” and 50 ng/ml of rat recombinant TNFα (Sigma-Aldrich, St, Louis, MO, USA). Both compounds were added into the cell culture medium for 24 h. We haven’t noticed any cell culture density changes or morphological alterations under inverted microscope (Fig. 2A). The expression analysis of genes coding mitochondrial fusion/fission proteins: opa1, mfn1, fis1, and mff (mitochondrial fission factor; MFF) revealed a decrease of opa1, mfn1, and, evident, but to be confirmed tendency to increase of the fission related fis1 gene indicating disturbed fission process in the mitochondria of RBE4 cells upon treatments with both factors (Fig. 2B). Besides Fis1 participation in the mitochondrial fission via interactions with the Drp1, or by prevention of mitochondrial fusion through the inhibition of Mfn2/Opa1, Fis1 participates in mitophagy through recruitment of TBC1D15/17 and Syntaxin17 to mitochondria [120]. Fis1 is also proposed to interact with BAP31, inciting apoptosis [121]. Thus, Fis1 may act an important role in mitochondrial alternations during HE.
The effect of ammonia and/or TNFα treatment (5 mM ammonium chloride; 50 ng/ml TNFα; 24 h; Sigma-Aldrich; St. Louis, MO, USA) on RBE4 cell culture morphology and growth (A), mitochondrial gene expression (B), mitochondrial membrane potential (MMP) (C), monolayer permeability for fluorescein isothiocyanate (FITC) dextran 40 kDa (D). Relative gene expression was analyzed using the real-time PCR method followed by ΔΔCt quantification analysis in relation to beta actin gene expression. Probes for opa1(#Rn00592200_m1), mf1 (#Rn00594496_m1), fis1(#Rn01480911_m1), mff (#Rn01400790_m1) and actb (Rn 0066789_m1) were purchased from Applied Biosystems, Waltham, USA. MMP was established using a Mitochondrial Membrane Potential kit, based on a JC-10 fluorescence probe, according to the manufacturer’s protocol (cat# MAK159, Sigma-Aldrich, Saint Louis, USA). Cells monolayer permeability for FITC-dextran (30 min exposition) was measured fluorometrically at 485/520 nm. Results are mean ± SD (n = 4); Two-way ANOVA test with Dunnett’s multiple comparisons test was performed using Graph Pad Software *p < 0.05 vs control; **p < 0.01 vs control; # 0.05 < p < 0.1 (tend toward significance) (panel B). One-way ANOVA test with Dunnett post-hoc test was performed using Graph Pad Software *p < 0.05 vs control; **p < 0.01 vs control; **p < 0.001 vs control (panel C and D)
The measurement of mitochondrial membrane potential (MMP) revealed engrossing observation. MMP was decreased in both ammonia-treated groups after a short 30’ exposition. In turn, MMP was not significantly affected by ammonia but reduced in both TNFα- treated groups after 24 h (Fig. 2C). The short exposition of ammonia exerts probably toxic effects mainly by disturbing the pH balance. Ammonia in solution is present as NH3 or NH4+. NH3 is a weak base in gaseous form, since NH4+ is a weak acid. Because ammonia pKa is relatively high (approximately 9.2) most of total ammonia at physiologic pH is NH4+ [122, 123]. Ammonia toxicity results from disruption of the H+ gradient across the inner membranes of mitochondria. Due to relative alkalinity of the mitochondrial pH as compared to the cytoplasmic pH ammonia exits the mitochondrial matrix along this gradient and binds to H+ in the inter-membrane space, thereby eliminating the H+ gradient necessary for ATP synthesis [124]. In addition, NH4+ could compete with K+ ions at the K+ binding site of K+-channels and affect excitability and membrane potential in neurons [125]. Whether the same applies to endothelial mitochondria is not clear. In turn, the effects of TNFα may be linked with TNFα receptor activation and further signals transducing. In the endothelium, TNFα induces inflammatory responses by enhancing adhesion molecule expression and cytokine secretion [126, 127].
To verify if ammonia/TNFα treatment and observed mitochondrial impairment disturb endothelium function we measured cell monolayer permeability to isothiocyanate FITC-dextran. Simultaneous ammonia/TNFα administration caused a significant increase in permeability indicative of a disturbed barrier function of the endothelial cells (Fig. 2D). Experiments demonstrated an almost fourfold increase in ROS content in all treated cells with an accompanying decrease of total antioxidant capacity (TAC) especially, after simultaneous ammonia and TNFα treatment (Fig. 3).
The effect of ammonia and/or TNFα treatment (5 mM ammonium chloride; 50 ng/ml TNFα; 24 h; Sigma-Aldrich; St. Louis, MO, USA) on the content of reactive oxygen species (ROS) (A) and total antioxidant capacity (TAC) (B). ROS levels were measured using the fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCF-DA) 5 μM for 30 min at 37 °C. The fluorescence of cells was detected using Fluorescence Microplate Reader FLUOstar OMEGA (BMG Labtech, Ortenberg, Germany) with an excitation wavelength of 485/20 nm and emission wavelength of 528/20 nm. TAC was measured using the TAC Assay kit (cat# MAK187, Sigma-Aldrich, Saint Louise, USA) according to the manufacturer's instructions by the estimation of the capacity of the total antioxidants in the sample to convert Cu2+ in its reduced form, Cu+ which chelates with a colorimetric probe, giving a broad absorbance peak at ∼570 nm. Results are mean ± SD (n = 4); One-way ANOVA test with Dunnett post-hoc test was performed using Graph Pad Software **p < 0.01 vs control; ***p < 0.001 vs control
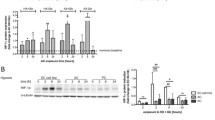
We verified the morphology of mitochondria in cerebral vessels isolated from rats with hyperammonemia (OA) and thioacetamide (TAA)—induced acute liver failure. Both models were performed on male Sprague–Dawley rats (Tac: Cmd: SD, weight 200–220 g) supplied by the Animal House of Mossakowski Medical Research Centre, Warsaw, Poland (Approval no. 57/2015 (14 May 2015 from the 4th Local Ethics Committee for Animal Experimentation, Warsaw, Poland, as compliant with Polish Law). Briefly, hyperammonemia (OA) was induced by ip. injections of ammonium acetate (600 mg per kg) at 12 h intervals for three days. Acute liver failure was induced by thioacetamide (TAA) ip. administration (300 mg per kg at 24 intervals for three days). The control (sham) group received 0.3 mL of 0.9% NaCl (ip. for 3 days). Brain cortex microvessels were isolated on saccharose gradient as described earlier [63].
Electron microscopy documented enlarged mitochondria in the brain endothelial cells of OA and TAA rats suggesting mitochondrial swelling that occurs in both models (Fig. 4A), however, the mitochondria aspect ratio (width/length) evaluated using Digimizer Software (MedCalc Software Ltd., Ostend, Belgium) was unchanged. The mitochondrial membrane potential measurement in rat brain microvessels revealed a tendency toward a decrease in both experimental models which implies mitochondrial dysfunction leading to brain vessels energy depletion (Fig. 4B). The expression analysis of genes coding key fusion/fission proteins: opa1, mfn, fis1, and mff indicated possible increased fission of OA cerebral mitochondria and a decreased fusion of cerebral mitochondria from TAA rats, but further experiments are needed to prove this phenomenon (Fig. 4D), since gene expression changes are not necessarily reflected at the protein level.
Mitochondria of cerebral vessels isolated from the brains of rats with hyperammonemia (OA) and thioacetamide (TAA)-induced acute liver failure. The length of the mitochondria in the brain vessels endothelium in the electron microscopy images was determined by quantitative evaluation using Digimizer Image Analysis Software (MedCalc Software Ltd, Ostend, Belgium) (A). Mitochondrial membrane potential (MMP) in cerebral vessels mitochondria of control, (OA), and (TAA)-administered rats was determined using Mitochondrial Membrane Potential kit (cat# MAK159, Sigma-Aldrich, Saint Louis, USA) (B). Representative electron microscopy images of cerebral vessel mitochondria and confocal microscopic image of vessels with von Willebrand factor immunostaining (C). Relative mitochondrial gene expression was measured using the real-time PCR method followed by ΔΔCt quantification analysis to beta-actin gene expression. Details of probes used are listed in the legend to Fig. 3 (D). All results are mean ± SD (n = 4); Two-way ANOVA test with Dunnett’s multiple comparisons test was performed using Graph Pad Software *p < 0.05 vs control; **p < 0.01 vs control (panel B). One-way ANOVA test with Dunnett post-hoc test was performed using Graph Pad Software *p < 0.05; **p < 0.01; ***p < 0.001 (panel C and D)
Roles of Mitochondrial Membrane Potential (Δψ) and Calcium for ROS Generation by the Mitochondria
The issues circumscribed by the title of this section have not been elaborated in our laboratory, but, definitely deserve comment. The role of mitochondrial membrane potential (Δψ) in ROS production is ambiguous. The complexity of the issue is underscored by findings variably associating ROS production with hyperpolarization and depolarization, likely depending on pharmacologically active agents and substrates being used, and the respiratory and cytoplasmic redox potential of the mitochondria. Selective MitoK+ATP openers that decrease Δψ in the cerebrovasculature do not increase ROS production [128].
The role of mitochondrial Δψ and ROS production in vascular dysfunction derives from data demonstrating that compared to healthy controls, arterioles and circulating mononuclear cells from patients with obesity and type 2 diabetes are characterized by mitochondrial membrane hyperpolarization (more negative Δψ), reduced mitochondrial mass, and greater ROS production [129,130,131]. Importantly, differences in mitochondrial membrane potential were observed as well. Mitochondrial alterations including membrane hyperpolarization reduced NO bioavailability and affected vascular function [103, 104]. In the former study, mitochondrial ROS production in monocytes was negatively correlated with artery flow-mediated dilation [129], a marker of endothelial function in humans.
Intracellular Ca2+ is essential for maintaining endothelium integrity and function [132]. In particular, the interaction of mitochondria with the endoplasmic reticulum is central in regulating intracellular Ca2+ levels, and this process directly involves mitochondrial Ca2+ uptake and cycling [133]. In addition to intracellular Ca2+ homeostasis control, mitochondrial Ca2+ levels play obvious roles in mitochondrial metabolism, cell signaling, biogenesis, and morphology [134], processes pertinent to optimal vascular function. In endothelial cells, increases in intracellular Ca2+ lead to eNOS activation and subsequent NO production [132]. As previously documented by our group, high levels of ammonia evoked a durable decrease of the basal intracellular Ca2+ level in RBE4 cells [135], which may contribute to cerebral vascular endothelial dysfunction associated with hyperammonemia and/or HE. The presence of ammonia-sensitive intracellular Ca2+ reservoirs has been previously described in bovine aortic endothelial cells [136]. However, the details of the evolution of Ca2+ to BBB dysfunction associated with HE have not been unraveled in those studies. Of note, increased Ca2+ efflux from brain mitochondria was observed in rats injected with ammonia [137].
Whether and how Ca2+ fluxes or other intermediary events occurring at the molecular or cellular level affect the function of cerebral blood vessels are translated into their functioning are intriguing questions worth further investigation.
Conclusions
Investigations carried out to date revealed numerous potential links between various aspects of mitochondrial dynamics in rat brain endothelial cells and BBB dysfunction and its role in the pathogenesis of HE. However, the picture is far from being complete and the roads to the goal are filled with methodological obstacles. In terms of mitochondrial dynamics itself, the evidence regarding responses of its executors is still in a very preliminary stage. In terms of its link to BBB disruption, the status is obscured by a great model-to-model variability, which makes it difficult to translate the experimental results to clinical observations. Undoubtedly, an extension of the present knowledge on endothelial ammonia metabolism and how it affects the endothelial mitochondria is needed. Issues such as the role of glutamine metabolism and transport, operation of the glutamate/glutamine cycle, very well described in astrocytes, remain almost unaddressed concerning brain endothelial cells. Raising the level of knowledge of the above aspects of metabolism in HE-affected endothelial mitochondria to that already acquired about astrocytic mitochondria should help to unravel the secrets of BBB disruption and brain edema in HE.
Data Availability
The data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Change history
08 October 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11064-022-03780-0
Abbreviations
- ALF:
-
Acute liver failure
- BBB:
-
Blood–brain barrier
- BDL:
-
Bile duct ligation
- BH4:
-
Tetrahydrobiopterin
- DCFH-DA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- HE:
-
Hepatic encephalopathy
- MMP:
-
Mitochondrial membrane potential
- MMP9:
-
Matrix metalloproteinase 9
- mPTP:
-
Mitochondrial permeability transition pore
- NO:
-
Nitric oxide
- OA:
-
Ammonium acetate/here used to refer to a rat model of hyperammonaemia
- ROS:
-
Reactive oxygen species
- TAA:
-
Thioacetamide
- TAC:
-
Total antioxidant capacity
- TJs:
-
Tight junctions
- VE- Cadherin:
-
Vascular endothelial-cadherin
- ZO:
-
Zonula occludens
References
Prakash R, Mullen KD (2010) Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol 7:515–525
Albrecht J, Jones EA (1999) Hepatic encephalopathy: molecular mechanisms underlying the clinical syndrome. J Neurol Sci 170:138–146
Felipo V, Butterworth RF (2002) Neurobiology of ammonia. Prog Neurobiol 67:259–279
Blei AT (2008) Brain edema in acute liver failure. Crit Care Clin 24(99–114):ix
Liotta EM, Kimberly WT (2020) Cerebral edema and liver disease: Classic perspectives and contemporary hypotheses on mechanism. Neurosci Lett 721:134818
Shawcross DL, Sharifi Y, Canavan JB, Yeoman AD, Abeles RD, Taylor NJ, Auzinger G, Bernal W, Wendon JA (2011) Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol 54:640–649
Milewski K, Oria M (2016) What we know: the inflammatory basis of hepatic encephalopathy. Metab Brain Dis 31(6):1239–1247
Cabrera-Pastor A, Llansola M, Montoliu C, Malaguarnera M, Balzano T, Taoro-Gonzalez L, Garcia-Garcia R, Mangas-Losada A, Izquierdo-Altarejos P, Arenas YM, Leone P, Felipo V (2019) Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: underlying mechanisms and therapeutic implications. Acta Physiol 226:e13270
Bosoi CR, Rose CF (2013) Brain edema in acute liver failure and chronic liver disease: similarities and differences. Neurochem Int 62:446–457
Chavarria L, Cordoba J (2014) Magnetic resonance of the brain in chronic and acute liver failure. Metab Brain Dis 29:937–944
Norenberg MD (1987) The role of astrocytes in hepatic encephalopathy. Neurochem Pathol 6:13–33
Albrecht J, Zielinska M, Norenberg MD (2010) Glutamine as a mediator of ammonia neurotoxicity: a critical appraisal. Biochem Pharmacol 80:1303–1308
Haussinger D, Schliess F (2008) Pathogenetic mechanisms of hepatic encephalopathy. Gut 57:1156–1165
Albrecht J, Norenberg MD (2006) Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology 44:788–794
Rama Rao KV, Jayakumar AR, Norenberg MD (2005) Role of oxidative stress in the ammonia-induced mitochondrial permeability transition in cultured astrocytes. Neurochem Int 47:31–38
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W (2016) ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev 2016:4350965
Tsujimoto Y, Shimizu S (2007) Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12:835–840
Rama Rao KV, Jayakumar AR, Norenberg MD (2003) Induction of the mitochondrial permeability transition in cultured astrocytes by glutamine. Neurochem Int 43:517–523
Alvarez VM, Rama Rao KV, Brahmbhatt M, Norenberg MD (2011) Interaction between cytokines and ammonia in the mitochondrial permeability transition in cultured astrocytes. J Neurosci Res 89:2028–2040
Bjerring PN, Hauerberg J, Frederiksen HJ, Jorgensen L, Hansen BA, Tofteng F, Larsen FS (2008) Cerebral glutamine concentration and lactate-pyruvate ratio in patients with acute liver failure. Neurocrit Care 9:3–7
Scott TR, Kronsten VT, Hughes RD, Shawcross DL (2013) Pathophysiology of cerebral oedema in acute liver failure. World J Gastroenterol 19:9240–9255
Jayakumar AR, Rama Rao KV, Norenberg MD (2015) Neuroinflammation in hepatic encephalopathy: mechanistic aspects. J Clin Exp Hepatol 5:S21-28
Hadjihambi A, Arias N, Sheikh M, Jalan R (2018) Hepatic encephalopathy: a critical current review. Hep Intl 12:135–147
Risau W, Wolburg H (1990) Development of the blood-brain barrier. Trends Neurosci 13:174–178
Langen UH, Ayloo S, Gu C (2019) Development and cell biology of the Blood-Brain Barrier. Annu Rev Cell Dev Biol 35:591–613
Almutairi MM, Gong C, Xu YG, Chang Y, Shi H (2016) Factors controlling permeability of the blood-brain barrier. Cell Mol Life Sci 73:57–77
del Zoppo GJ, Milner R (2006) Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol 26:1966–1975
Osada T, Gu YH, Kanazawa M, Tsubota Y, Hawkins BT, Spatz M, Milner R, del Zoppo GJ (2011) Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by beta(1)-integrins. J Cereb Blood Flow Metab 31:1972–1985
Daneman R, Zhou L, Kebede AA, Barres BA (2010) Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468:562–566
Armulik A, Genove G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21:193–215
Castro V, Skowronska M, Lombardi J, He J, Seth N, Velichkovska M, Toborek M (2018) Occludin regulates glucose uptake and ATP production in pericytes by influencing AMP-activated protein kinase activity. J Cereb Blood Flow Metab 38:317–332
Grubb S, Lauritzen M, Aalkjaer C (2021) Brain capillary pericytes and neurovascular coupling. Comp Biochem Physiol A 254:110893
Kato M, Hughes RD, Keays RT, Williams R (1992) Electron microscopic study of brain capillaries in cerebral edema from fulminant hepatic failure. Hepatology 15:1060–1066
Cauli O, Lopez-Larrubia P, Rodrigo R, Agusti A, Boix J, Nieto-Charques L, Cerdan S, Felipo V (2011) Brain region-selective mechanisms contribute to the progression of cerebral alterations in acute liver failure in rats. Gastroenterology 140:638–645
Yamamoto S, Nguyen JH (2006) TIMP-1/MMP-9 imbalance in brain edema in rats with fulminant hepatic failure. J Surg Res 134:307–314
Chen F, Ohashi N, Li W, Eckman C, Nguyen JH (2009) Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure. Hepatology 50:1914–1923
Lv S, Song HL, Zhou Y, Li LX, Cui W, Wang W, Liu P (2010) Tumour necrosis factor-alpha affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int 30:1198–1210
Traber PG, Dal Canto M, Ganger DR, Blei AT (1987) Electron microscopic evaluation of brain edema in rabbits with galactosamine-induced fulminant hepatic failure: ultrastructure and integrity of the blood-brain barrier. Hepatology 7:1272–1277
Potvin M, Finlayson MH, Hinchey EJ, Lough JO, Goresky CA (1984) Cerebral abnormalities in hepatectomized rats with acute hepatic coma. Lab Investig 50:560–564
Kristiansen RG, Lindal S, Myreng K, Revhaug A, Ytrebo LM, Rose CF (2010) Neuropathological changes in the brain of pigs with acute liver failure. Scand J Gastroenterol 45:935–943
Wright G, Davies NA, Shawcross DL, Hodges SJ, Zwingmann C, Brooks HF, Mani AR, Harry D, Stadlbauer V, Zou Z, Williams R, Davies C, Moore KP, Jalan R (2007) Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology 45:1517–1526
Maly IP, Landmann L (2008) Bile duct ligation in the rat causes upregulation of ZO-2 and decreased colocalization of claudins with ZO-1 and occludin. Histochem Cell Biol 129:289–299
Alexander B, Li X, Benjamin IS, Segal MB, Sherwood R, Preston JE (2000) A quantitative evaluation of the permeability of the blood brain barrier of portacaval shunted rats. Metab Brain Dis 15:93–103
McMillin MA, Frampton GA, Seiwell AP, Patel NS, Jacobs AN, DeMorrow S (2015) TGFbeta1 exacerbates blood-brain barrier permeability in a mouse model of hepatic encephalopathy via upregulation of MMP9 and downregulation of claudin-5. Lab Investig 95:903–913
Grant S, McMillin M, Frampton G, Petrescu AD, Williams E, Jaeger V, Kain J, DeMorrow S (2018) Direct comparison of the thioacetamide and azoxymethane models of Type A hepatic encephalopathy in mice. Gene Expr 18:171–185
Obara-Michlewska M, Ding F, Popek M, Verkhratsky A, Nedergaard M, Zielinska M, Albrecht J (2018) Interstitial ion homeostasis and acid-base balance are maintained in oedematous brain of mice with acute toxic liver failure. Neurochem Int 118:286–291
Wang W, Lv S, Zhou Y, Fu J, Li C, Liu P (2011) Tumor necrosis factor-alpha affects blood-brain barrier permeability in acetaminophen-induced acute liver failure. Eur J Gastroenterol Hepatol 23:552–558
Estevao C, Bowers CE, Luo D, Sarker M, Hoeh AE, Frudd K, Turowski P, Greenwood J (2021) CCL4 induces inflammatory signalling and barrier disruption in the neurovascular endothelium. Brain Behav Immunity Health 18:100370
Knudsen GM, Poulsen HE, Paulson OB (1988) Blood-brain barrier permeability in galactosamine-induced hepatic encephalopathy. No evidence for increased GABA-transport. J Hepatol 6:187–192
Lo WD, Ennis SR, Goldstein GW, McNeely DL, Betz AL (1987) The effects of galactosamine-induced hepatic failure upon blood-brain barrier permeability. Hepatology 7:452–456
Dixit V, Chang TM (1990) Brain edema and the blood brain barrier in galactosamine-induced fulminant hepatic failure rats. An animal model for evaluation of liver support systems. ASAIO Trans 36:21–27
Horowitz ME, Schafer DF, Molnar P, Jones EA, Blasberg RG, Patlak CS, Waggoner J, Fenstermacher JD (1983) Increased blood-brain transfer in a rabbit model of acute liver failure. Gastroenterology 84:1003–1011
Liu L, Miao M, Chen Y, Wang Z, Sun B, Liu X (2018) Altered function and expression of ABC Transporters at the Blood-Brain barrier and increased brain distribution of phenobarbital in acute liver failure mice. Front Pharmacol 9:190
Traber P, DalCanto M, Ganger D, Blei AT (1989) Effect of body temperature on brain edema and encephalopathy in the rat after hepatic devascularization. Gastroenterology 96:885–891
Miah MK, Shaik IH, Bickel U, Mehvar R (2015) Effects of Pringle maneuver and partial hepatectomy on the pharmacokinetics and blood-brain barrier permeability of sodium fluorescein in rats. Brain Res 1618:249–260
Zaki AE, Ede RJ, Davis M, Williams R (1984) Experimental studies of blood brain barrier permeability in acute hepatic failure. Hepatology 4:359–363
Vairappan B, Sundhar M, Srinivas BH (2019) Resveratrol restores neuronal tight junction proteins through correction of ammonia and inflammation in CCl4-induced cirrhotic mice. Mol Neurobiol 56:4718–4729
Baek SY, Lee EH, Oh TW, Do HJ, Kim KY, Park KI, Kim YW (2020) Network pharmacology-based approaches of rheum undulatum linne and glycyrriza uralensis fischer imply their regulation of liver failure with hepatic encephalopathy in Mice. Biomolecules 10:437
Shaik IH, Miah MK, Bickel U, Mehvar R (2013) Effects of short-term portacaval anastomosis on the peripheral and brain disposition of the blood-brain barrier permeability marker sodium fluorescein in rats. Brain Res 1531:84–93
Laursen H, Schroder H, Westergaard E (1975) The effect of portocaval anastomosis on the permeability to horseradish peroxidase of cerebral vessels of the rat. Acta Pathol Microbiol Scand Sect A 83:266–268
Or M, Devriendt N, Kitshoff AM, Peremans K, Vandermeulen E, Paepe D, Polis I, Martle V, de Rooster H (2017) Ammonia concentrations in arterial blood, venous blood, and cerebrospinal fluid of dogs with and without congenital extrahepatic portosystemic shunts. Am J Vet Res 78:1313–1318
Eizayaga F, Scorticati C, Prestifilippo JP, Romay S, Fernandez MA, Castro JL, Lemberg A, Perazzo JC (2006) Altered blood-brain barrier permeability in rats with prehepatic portal hypertension turns to normal when portal pressure is lowered. World J Gastroenterol 12:1367–1372
Quinn M, McMillin M, Galindo C, Frampton G, Pae HY, DeMorrow S (2014) Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Diges Liver Dis 46:527–534
Chavarria L, Alonso J, Rovira A, Cordoba J (2011) Neuroimaging in acute liver failure. Neurochem Int 59:1175–1180
Lockwood AH, Yap EW, Wong WH (1991) Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J Cereb Blood Flow Metab 11:337–341
Keiding S, Sorensen M, Bender D, Munk OL, Ott P, Vilstrup H (2006) Brain metabolism of 13N-ammonia during acute hepatic encephalopathy in cirrhosis measured by positron emission tomography. Hepatology 43:42–50
Goldbecker A, Buchert R, Berding G, Bokemeyer M, Lichtinghagen R, Wilke F, Ahl B, Weissenborn K (2010) Blood-brain barrier permeability for ammonia in patients with different grades of liver fibrosis is not different from healthy controls. J Cereb Blood Flow Metab 30:1384–1393
de Boer AG, Gaillard PJ (2006) Blood-brain barrier dysfunction and recovery. J Neural Transm 113:455–462
Jiang W, Desjardins P, Butterworth RF (2009) Direct evidence for central proinflammatory mechanisms in rats with experimental acute liver failure: protective effect of hypothermia. J Cereb Blood Flow Metab 29:944–952
Balzano T, Dadsetan S, Forteza J, Cabrera-Pastor A, Taoro-Gonzalez L, Malaguarnera M, Gil-Perotin S, Cubas-Nunez L, Casanova B, Castro-Quintas A, Ponce-Mora A, Arenas YM, Leone P, Erceg S, Llansola M, Felipo V (2020) Chronic hyperammonemia induces peripheral inflammation that leads to cognitive impairment in rats: reversed by anti-TNF-alpha treatment. J Hepatol 73:582–592
Butterworth RF (2019) Hepatic encephalopathy in cirrhosis: pathology and pathophysiology. Drugs 79:17–21
Tsao N, Hsu HP, Wu CM, Liu CC, Lei HY (2001) Tumour necrosis factor-alpha causes an increase in blood-brain barrier permeability during sepsis. J Med Microbiol 50:812–821
Wright G, Shawcross D, Olde Damink SW, Jalan R (2007) Brain cytokine flux in acute liver failure and its relationship with intracranial hypertension. Metab Brain Dis 22:375–388
Jalan R, Olde Damink SW, Hayes PC, Deutz NE, Lee A (2004) Pathogenesis of intracranial hypertension in acute liver failure: inflammation, ammonia and cerebral blood flow. J Hepatol 41:613–620
Culic O, Gruwel ML, Schrader J (1997) Energy turnover of vascular endothelial cells. Am J Physiol 273:C205-213
Freed JK, Gutterman DD (2013) Mitochondrial reactive oxygen species and vascular function: less is more. Arterioscler Thromb Vasc Biol 33:673–675
Zhang DX, Gutterman DD (2007) Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292:H2023-2031
Kluge MA, Fetterman JL, Vita JA (2013) Mitochondria and endothelial function. Circ Res 112:1171–1188
Quintero M, Colombo SL, Godfrey A, Moncada S (2006) Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci USA 103:5379–5384
Skowrońska M, Zielińska M, Wójcik-Stanaszek L, Ruszkiewicz J, Milatovic D, Aschner M, Albrecht J (2012) Ammonia increases paracellular permeability of rat brain endothelial cells by a mechanism encompassing oxidative/nitrosative stress and activation of matrix metalloproteinases. J Neurochem 121:125–134
Jayakumar AR, Tong XY, Ospel J, Norenberg MD (2012) Role of cerebral endothelial cells in the astrocyte swelling and brain edema associated with acute hepatic encephalopathy. Neuroscience 218:305–316
Dhanda S, Sandhir R (2018) Blood-Brain Barrier permeability is exacerbated in experimental model of hepatic encephalopathy via MMP-9 activation and downregulation of tight junction proteins. Mol Neurobiol 55:3642–3659
Milewski K, Czarnecka AM, Albrecht J, Zielinska M (2021) Decreased expression and uncoupling of endothelial nitric oxide synthase in the cerebral cortex of rats with thioacetamide-induced acute liver failure. Int J Mol Sci 22:6662
Hilgier W, Anderzhanova E, Oja SS, Saransaari P, Albrecht J (2003) Taurine reduces ammonia- and N-methyl-D-aspartate-induced accumulation of cyclic GMP and hydroxyl radicals in microdialysates of the rat striatum. Eur J Pharmacol 468:21–25
Genesca J, Gonzalez A, Segura R, Catalan R, Marti R, Varela E, Cadelina G, Martinez M, Lopez-Talavera JC, Esteban R, Groszmann RJ, Guardia J (1999) Interleukin-6, nitric oxide, and the clinical and hemodynamic alterations of patients with liver cirrhosis. Am J Gastroenterol 94:169–177
Zielińska M, Ruszkiewicz J, Hilgier W, Fresko I, Albrecht J (2011) Hyperammonemia increases the expression and activity of the glutamine/arginine transporter y+ LAT2 in rat cerebral cortex: implications for the nitric oxide/cGMP pathway. Neurochem Int 58:190–195
Hilgier W, Fresko I, Klemenska E, Beresewicz A, Oja SS, Saransaari P, Albrecht J, Zielinska M (2009) Glutamine inhibits ammonia-induced accumulation of cGMP in rat striatum limiting arginine supply for NO synthesis. Neurobiol Dis 35:75–81
Rama Rao KV, Reddy PV, Tong X, Norenberg MD (2010) Brain edema in acute liver failure: inhibition by L-histidine. Am J Pathol 176:1400–1408
Gorg B, Karababa A, Schutz E, Paluschinski M, Schrimpf A, Shafigullina A, Castoldi M, Bidmon HJ, Haussinger D (2019) O-GlcNAcylation-dependent upregulation of HO1 triggers ammonia-induced oxidative stress and senescence in hepatic encephalopathy. J Hepatol 71:930–941
Okada T, Watanabe Y, Brusilow SW, Traystman RJ, Koehler RC (2000) Interaction of glutamine and arginine on cerebrovascular reactivity to hypercapnia. Am J Physiol Heart Circ Physiol 278:H1577-1584
Czarnecka A, Aleksandrowicz M, Jasinski K, Jazwiec R, Kalita K, Hilgier W, Zielinska M (2018) Cerebrovascular reactivity and cerebral perfusion of rats with acute liver failure: role of L-glutamine and asymmetric dimethylarginine in L-arginine-induced response. J Neurochem 147:692–704
Cassuto J, Dou H, Czikora I, Szabo A, Patel VS, Kamath V, Belin de Chantemele E, Feher A, Romero MJ, Bagi Z (2014) Peroxynitrite disrupts endothelial caveolae leading to eNOS uncoupling and diminished flow-mediated dilation in coronary arterioles of diabetic patients. Diabetes 63:1381–1393
Katakam PV, Snipes JA, Steed MM, Busija DW (2012) Insulin-induced generation of reactive oxygen species and uncoupling of nitric oxide synthase underlie the cerebrovascular insulin resistance in obese rats. J Cereb Blood Flow Metab 32:792–804
Forstermann U, Munzel T (2006) Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113:1708–1714
Werner ER, Blau N, Thony B (2011) Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem J 438:397–414
Tinker A, Aziz Q, Thomas A (2014) The role of ATP-sensitive potassium channels in cellular function and protection in the cardiovascular system. Br J Pharmacol 171:12–23
Peng K, Hu J, Xiao J, Dan G, Yang L, Ye F, Zou Z, Cao J, Sai Y (2018) Mitochondrial ATP-sensitive potassium channel regulates mitochondrial dynamics to participate in neurodegeneration of Parkinson’s disease. Biochim Biophys Acta 1864:1086–1103
Daiber A (2010) Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochem Biophys Acta 1797:897–906
Reinehr R, Gorg B, Becker S, Qvartskhava N, Bidmon HJ, Selbach O, Haas HL, Schliess F, Haussinger D (2007) Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. Glia 55:758–771
Kosenko E, Kaminsky Y, Kaminsky A, Valencia M, Lee L, Hermenegildo C, Felipo V (1997) Superoxide production and antioxidant enzymes in ammonia intoxication in rats. Free Radic Res 27:637–644
Hilgier W, Wegrzynowicz M, Ruszkiewicz J, Oja SS, Saransaari P, Albrecht J (2010) Direct exposure to ammonia and hyperammonemia increase the extracellular accumulation and degradation of astroglia-derived glutathione in the rat prefrontal cortex. Toxicol Sci 117:163–168
Jiang W, Desjardins P, Butterworth RF (2009) Minocycline attenuates oxidative/nitrosative stress and cerebral complications of acute liver failure in rats. Neurochem Int 55:601–605
Sushma S, Dasarathy S, Tandon RK, Jain S, Gupta S, Bhist MS (1992) Sodium benzoate in the treatment of acute hepatic encephalopathy: a double-blind randomized trial. Hepatology 16:138–144
Oldendorf WH, Cornford ME, Brown WJ (1977) The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol 1:409–417
Al-Mehdi AB, Pastukh VM, Swiger BM, Reed DJ, Patel MR, Bardwell GC, Pastukh VV, Alexeyev MF, Gillespie MN (2012) Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci Signaling 5:ra47
Murphy E (2015) Solving mitochondrial mysteries. J Mol Cell Cardiol 78:1–2
Moore AS, Holzbaur ELF (2018) Mitochondrial-cytoskeletal interactions: dynamic associations that facilitate network function and remodeling. Curr Opin Physiol 3:94–100
Srinivasan S, Guha M, Kashina A, Avadhani NG (2017) Mitochondrial dysfunction and mitochondrial dynamics—the cancer connection. Biochim Biophys Acta 1858:602–614
Busija DW, Rutkai I, Dutta S, Katakam PV (2016) Role of mitochondria in cerebral vascular function: energy production, cellular protection, and regulation of vascular tone. Compr Physiol 6:1529–1548
Ordys BB, Launay S, Deighton RF, McCulloch J, Whittle IR (2010) The role of mitochondria in glioma pathophysiology. Mol Neurobiol 42:64–75
Chakravorty A, Jetto CT, Manjithaya R (2019) Dysfunctional mitochondria and mitophagy as drivers of Alzheimer’s disease pathogenesis. Front Aging Neurosci 11:311
Bhatti JS, Bhatti GK, Reddy PH (2017) Mitochondrial dysfunction and oxidative stress in metabolic disorders—a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta 1863:1066–1077
Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A (2008) Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294:H2121-2128
Kovac S, Dinkova Kostova AT, Herrmann AM, Melzer N, Meuth SG, Gorji A (2017) Metabolic and homeostatic changes in seizures and acquired epilepsy-mitochondria, calcium dynamics and reactive oxygen species. Int J Mol Sci 18:1935
Doll DN, Hu H, Sun J, Lewis SE, Simpkins JW, Ren X (2015) Mitochondrial crisis in cerebrovascular endothelial cells opens the blood-brain barrier. Stroke 46:1681–1689
Drouin-Ouellet J, Sawiak SJ, Cisbani G, Lagace M, Kuan WL, Saint-Pierre M, Dury RJ, Alata W, St-Amour I, Mason SL, Calon F, Lacroix S, Gowland PA, Francis ST, Barker RA, Cicchetti F (2015) Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: potential implications for its pathophysiology. Ann Neurol 78:160–177
Gray MT, Woulfe JM (2015) Striatal blood-brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab 35:747–750
Kirk J, Plumb J, Mirakhur M, McQuaid S (2003) Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. J Pathol 201:319–327
Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14:133–150
Ihenacho UK, Meacham KA, Harwig MC, Widlansky ME, Hill RB (2021) Mitochondrial fission protein 1: emerging roles in organellar form and function in health and disease. Front Endocrinol 12:660095
Iwasawa R, Mahul-Mellier AL, Datler C, Pazarentzos E, Grimm S (2011) Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. EMBO J 30:556–568
Bosoi CR, Rose CF (2009) Identifying the direct effects of ammonia on the brain. Metab Brain Dis 24:95–102
Hamm LL, Nakhoul N, Hering-Smith KS (2015) Acid-base homeostasis. Clin J Am Soc Nephrol 10:2232–2242
Cooper AJ, Plum F (1987) Biochemistry and physiology of brain ammonia. Physiol Rev 67:440–519
Hertz L, Song D, Peng L, Chen Y (2017) Multifactorial effects on different types of brain cells contribute to ammonia toxicity. Neurochem Res 42:721–736
Li M, van Esch B, Henricks PAJ, Garssen J, Folkerts G (2018) Time and concentration dependent effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor alpha-induced endothelial activation. Front Pharmacol 9:233
Xia P, Gamble JR, Rye KA, Wang L, Hii CS, Cockerill P, Khew-Goodall Y, Bert AG, Barter PJ, Vadas MA (1998) Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci USA 95:14196–14201
Katakam PV, Wappler EA, Katz PS, Rutkai I, Institoris A, Domoki F, Gaspar T, Grovenburg SM, Snipes JA, Busija DW (2013) Depolarization of mitochondria in endothelial cells promotes cerebral artery vasodilation by activation of nitric oxide synthase. Arterioscler Thromb Vasc Biol 33:752–759
Kizhakekuttu TJ, Wang J, Dharmashankar K, Ying R, Gutterman DD, Vita JA, Widlansky ME (2012) Adverse alterations in mitochondrial function contribute to type 2 diabetes mellitus-related endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol 32:2531–2539
Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, Vita JA (2011) Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124:444–453
Widlansky ME, Wang J, Shenouda SM, Hagen TM, Smith AR, Kizhakekuttu TJ, Kluge MA, Weihrauch D, Gutterman DD, Vita JA (2010) Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl Res 156:15–25
Zhao Y, Vanhoutte PM, Leung SW (2015) Vascular nitric oxide: Beyond eNOS. J Pharmacol Sci 129:83–94
Kannurpatti SS (2017) Mitochondrial calcium homeostasis: Implications for neurovascular and neurometabolic coupling. J Cereb Blood Flow Metab 37:381–395
Szabadkai G, Duchen MR (2008) Mitochondria: the hub of cellular Ca2+ signaling. Physiology 23:84–94
Konopacka A, Zielinska M, Albrecht J (2008) Ammonia inhibits the C-type natriuretic peptide-dependent cyclic GMP synthesis and calcium accumulation in a rat brain endothelial cell line. Neurochem Int 52:1160–1166
Danthuluri NR, Kim D, Brock TA (1990) Intracellular alkalinization leads to Ca2+ mobilization from agonist-sensitive pools in bovine aortic endothelial cells. J Biol Chem 265:19071–19076
Kosenko E, Kaminsky Y, Stavroskaya IG, Felipo V (2000) Alteration of mitochondrial calcium homeostasis by ammonia-induced activation of NMDA receptors in rat brain in vivo. Brain Res 880:139–146
Acknowledgements
The authors thank Professor Jan Albrecht for his critical reading of the manuscript.
Funding
This study was supported by the National Science Centre of the Republic of Poland (NCN) Grant number: 2015/19/B/NZ4/01902 and the statutory MMRI funds No.13.
Author information
Authors and Affiliations
Contributions
MZ and KM conceived and designed the experiments; KM and KO-G performed the experiments and analyzed the data; MZ and KM wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Milewski, K., Orzeł-Gajowik, K. & Zielińska, M. Mitochondrial Changes in Rat Brain Endothelial Cells Associated with Hepatic Encephalopathy: Relation to the Blood–Brain Barrier Dysfunction. Neurochem Res 49, 1489–1504 (2024). https://doi.org/10.1007/s11064-022-03698-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03698-7