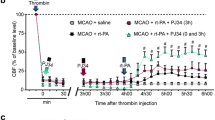
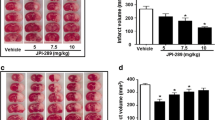
Abstract
Stroke is one of the leading causes of morbidity and mortality accompanied by blood supply loss to a particular brain area. Several mechanistic approaches such as inhibition of poly (ADP-ribose) polymerase, therapies against tissue thrombosis, and neutrophils lead to stroke’s therapeutic intervention. Evidence obtained with the poly (ADP-ribose) polymerase (PARP) inhibition and animals having a deficiency of PARP enzymes; represented the role of PARP in cerebral stroke, ischemia/reperfusion, and neurotrauma. PARP is a nuclear enzyme superfamily with various isoforms, each with different structural domains and functions, and out of all, PARP-1 is the best-characterized member. It has been shown to perform multiple physiological as well as pathological processes, including its role in inflammation, oxidative stress, apoptosis, and mitochondrial dysfunction. The enzyme interacts with NF-κB, p53, and other transcriptional factors to regulate survival and cell death and modulates multiple downstream signaling pathways. Clinical trials have also been conducted using PARP inhibitors for numerous disorders and have shown positive results. However, additional information is yet to be established for the therapeutic intervention of PARP inhibitors in stroke. These agents’ utilization appears to be challenging due to their unknown potential long-term side effects. PARP activity increased during ischemia, but its inhibition provided significant neuroprotection. Despite the increased interest in PARP as a pharmacological modulator for novel therapeutic therapies, the current review focused on stroke and poly ADP-ribosylation.



Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- ADP-ribose:
-
5′-ADP-3″-deoxypent-2″-enofuranose
- AIF:
-
Apoptosis-inducing factor
- ATP:
-
Adenosine triphosphate
- BRCT:
-
Breast cancer susceptibility protein C terminus motif
- Ced-3:
-
Cell death protein 3
- DNA:
-
Deoxyribonucleic acid
- ICAM-1:
-
Intercellular adhesion molecule-1
- INO-1001:
-
Inotek’s isoindolinone
- iNOS:
-
Inducible nitric oxide synthase
- IRI:
-
Ischemia–reperfusion injury
- NADH:
-
Nicotinamide
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NMDA:
-
N-methyl-D-aspartate
- NMNAT:
-
Nicotinamide mononucleotide adenylyl transferase
- NMNT:
-
Nicotinamide mononucleotide
- NO:
-
Nitric oxide
- NPC:
-
Nuclear pore complex
- NPRT:
-
Nicotinamide phosphoribosyl transferase
- PARG:
-
Poly (ADP-ribose) glycohydrolase
- PARP:
-
Poly (ADP-ribose) polymerase
- PJ 34:
-
N-(6-oxo-5,6-dihydro-phenanthridin-2-yl)-N,N-dimethylacetamide
- PoCo:
-
Post-conditioning
- ROS:
-
Reactive oxygen species
- TANK:
-
Tankyrase
- TNF-α:
-
Tumor necrosis factor-alpha
- VPARP:
-
Vault PARP
References
Moroni F, Chiarugi A (2009) Post-ischemic brain damage: targeting PARP-1 within the ischemic neurovascular units as a realistic avenue to stroke treatment. FEBS J 276(1):36–45
Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7(1):1–1
Van der Worp HB, van Gijn J (2007) Acute ischemic stroke. N Eng J Med 357(6):572–579
Simpkins AN, Janowski M, Oz HS et al (2020) Biomarker application for precision medicine in stroke. Transl stroke Res 11(4):615–627
Crawford RS, Albadawi H, Atkins MD et al (2010) Postischemic poly (ADP-ribose) polymerase (PARP) inhibition reduces ischemia reperfusion injury in a hind-limb ischemia model. Surg 148(1):110–118
Klaidman L, Morales M, Kem S, Yang J, Chang ML, Adams JD Jr (2003) Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacol 69(3):150–157
Khan H, Grewal AK, Singh TG (2022) Pharmacological postconditioning by protocatechuic acid attenuates brain injury in ischemia-reperfusion (I/R) mice model: Implications of nuclear factor erythroid-2-related factor pathway. Neuroscience. https://doi.org/10.1016/j.neuroscience.2022.03.016
Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22(9):391–397
Maruta H, Matsumura N, Tanuma SI (1997) Role of (ADP-ribose)n catabolism in DNA repair. Biochem Biophys Res Commun 236(2):265–269
Weise J, Isenmann S, Bähr M (2001) Increased expression and activation of poly (ADP-ribose) polymerase (PARP) contribute to retinal ganglion cell death following rat optic nerve transection. Cell Death Differ 8(8):801–807
Morales J, Li L, Fattah FJ et al (2014) Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit Rev Eukaryot Gene Expr 24(1):15–28
Ciftci O, Ullrich O, Schmidt CA, Diestel A, Hass R (2001) Regulation of the nuclear proteasome activity in myelomonocytic human leukemia cells after adriamycin treatment. Blood 97(9):2830–2838
Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Soto M, Pérez JM (2006) Poly (ADP-ribose) polymerase-1 (PARP-1) inhibitors in cancer chemotherapy. Recent Pat Anti-cancer Drug Discov 1(1):39–53
Virág L (2005) Structure and function of poly (ADP-ribose) polymerase-1: role in oxidative stress-related pathologies. Curr Vasc Pharmacol 3(3):209–214
Hatachi G, Tsuchiya T, Miyazaki T et al (2014) The poly (adenosine diphosphate-ribose) polymerase inhibitor PJ34 reduces pulmonary ischemia-reperfusion injury in rats. Transplantation 98(6):618
Wang J, Hao L, Wang Y et al (2015) Inhibition of poly (ADP-ribose) polymerase and inducible nitric oxide synthase protects against ischemic myocardial damage by reduction of apoptosis. Mol Med Rep 11(3):1768–1776
Yi M, Dong B, Qin S, Chu Q, Wu K, Luo S (2019) Advances and perspectives of PARP inhibitors. Exp Hematol Oncol 8(1):1–2
Jagtap P, Szabó C (2005) Poly (ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov 4(5):421–440
Nguewa PA, Fuertes MA, Valladares B, Alonso C, Pérez JM (2005) Poly (ADP-ribose) polymerases: homology, structural domains and functions. Novel therapeutical applications. Prog Biophys Mol Boil 88(1):143–172
Virág L, Szabó C (2002) The therapeutic potential of poly (ADP-ribose) polymerase inhibitors. Pharmacol Rev 54(3):375–429
Khan H, Sharma K, Kumar A, Kaur A, Singh TG (2022) Therapeutic implications of cyclooxygenase (COX) inhibitors in ischemic injury. Inflamm Res 71(3):277–292
Hauschildt S, Scheipers P, Bessler WG, Mülsch A (1992) Induction of nitric oxide synthase in L929 cells by tumour-necrosis factor α is prevented by inhibitors of poly (ADP-ribose) polymerase. Biochem J 288(1):255–260
Simbulan-Rosenthal CM, Rosenthal DS, Hilz H et al (1996) The expression of poly (ADP-ribose) polymerase during differentiation-linked DNA replication reveals that it is a component of the multiprotein DNA replication complex. Biochemistry 35(36):11622–11633
Tentori L, Portarena I, Graziani G (2002) Potential clinical applications of poly (ADP-ribose) polymerase (PARP) inhibitors. Pharmacol Res 45(2):73–85
Augustin A, Spenlehauer C, Dumond H et al (2003) PARP-3 localizes preferentially to the daughter centriole and interferes with the G1/S cell cycle progression. J Cell Sci 116(8):1551–1562
Kickhoefer VA, Siva AC, Kedersha NL et al (1999) The 193-kD vault protein, VPARP, is a novel poly (ADP-ribose) polymerase. J Cell Biol 146(5):917–928
Amé JC, Spenlehauer C, de Murcia G (2004) The PARP superfamily. BioEssays 26:882–893
Smith S, de Lange T (1999) Cell cycle dependent localization of the telomeric PARP, tankyrase, to nuclear pore complexes and centrosomes. J Cell Sci 112(21):3649–3656
Amé JC, Rolli V, Schreiber V et al (1999) PARP-2, A novel mammalian DNA damage-dependent poly (ADP-ribose) polymerase. J Biol Chem 274(25):17860–17868
Cuzzocrea S (2005) Shock, inflammation and PARP. Pharmacol Res 52(1):72–82
Park SH, Loftus EV, Yang SK (2014) Appendiceal skip inflammation and ulcerative colitis. Dig Dis Sci 59(9):2050–2057
Geboes K (1994) From inflammation to lesion. Acta Gastroenterol Belg 57(5–6):273–284
Khan H, Gupta A, Singh TG, Kaur A (2021) Mechanistic insight on the role of leukotriene receptors in ischemic–reperfusion injury. Pharmacol Rep 73(5):1240–1254
Rainger GE, Rowley AF, Nash GB (1998) Adhesion-dependent release of elastase from human neutrophils in a novel, flow-based model: specificity of different chemotactic agents. Blood 92(12):4819–4827
Nathan C (2002) Points of control in inflammation. Nature 420(6917):846–852
Shaeter E, Beecham EJ, Covey JM, Kohn K, Potter M (1988) Activated neutrophils induce prolonged DNA damage in neighbouring cells. Carcinogenesis 9(12):2297–2304
Cronstein BN, Weissmann G (1993) The adhesion molecules of inflammation. Arthritis Rheum 36(2):147–157
Dorai T, Aggarwal BB (2004) Role of chemopreventive agents in cancer therapy. Cancer Lett 215(2):129–140
Hassa PO, Hottiger MO (2002) The functional role of poly (ADP-ribose) polymerase 1 as novel coactivator of NF-κB in inflammatory disorders. Cell Mol Life Sci CMLS 59(9):1534–1553
Zhang JN, Ma Y, Wei XY et al (2019) Remifentanil protects against lipopolysaccharide-induced inflammation through PARP-1/NF-κB signaling PATHWAY. Mediators Inflamm 31:2019
Sun M, Zhao Y, Gu Y, Xu C (2012) Anti-inflammatory mechanism of taurine against ischemic stroke is related to down-regulation of PARP and NF-κB. Amino Acids 42(5):1735–1747
Pazzaglia S, Pioli C (2020) Multifaceted role of PARP-1 in DNA repair and inflammation: pathological and therapeutic implications in cancer and non-cancer diseases. Cells 9(1):41
Hans CP, Feng Y, Naura AS et al (2009) Protective effects of PARP-1 knockout on dyslipidemia-induced autonomic and vascular dysfunction in ApoE−/− mice: effects on eNOS and oxidative stress. PLoS ONE 4(10):e7430
Ahmad A, de Camargo Vieira J, de Mello AH et al (2019) The PARP inhibitor olaparib exerts beneficial effects in mice subjected to cecal ligature and puncture and in cells subjected to oxidative stress without impairing DNA integrity: A potential opportunity for repurposing a clinically used oncological drug for the experimental therapy of sepsis. Pharmacol Res 145:104263
Khan H, Kashyap A, Kaur A, Singh TG (2020) Pharmacological postconditioning: a molecular aspect in ischemic injury. J Pharm Pharmacol 72(11):1513–1527
Virag L, Scott GS, Cuzzocrea S, Marmer D, Salzman AL, Szabo C (1998) Peroxynitrite-induced thymocyte apoptosis: the role of caspases and poly (ADP-ribose) synthetase (PARS) activation. Immunology 94:345–355
Fiorillo C, Ponziani V, Giannini L et al (2003) Beneficial effects of poly (ADP-ribose) polymerase inhibition against the reperfusion injury in heart transplantation. Free Radic Res 37(3):331–339
Tronov VA, Konstantinov EM (2000) Hydrogen peroxide-induced DNA repair and death of resting human blood lymphocytes. Biochem Biokhim 65(11):1279–1286
Thapliyal S, Singh T, Handu S, Bisht M, Kumari P, Arya P, Srivastava P, Gandham R (2021) A review on potential footprints of ferulic acid for treatment of neurological disorders. Neurochem Res 46(5):1043–1057
Keuss MJ, Hjerpe R, Hsia O et al (2019) Unanchored tri‐NEDD8 inhibits PARP‐1 to protect from oxidative stress‐induced cell death. EMBO J 38(6):e100024
Bürkle A (2001) Physiology and pathophysiology of poly (ADP-ribosyl) ation. BioEssays 23(9):795–806
Grewal AK, Singh N, Singh TG (2019) Effects of resveratrol postconditioning on cerebral ischemia in mice: role of the sirtuin-1 pathway. Can J Physiol Pharmacol 97(11):1094–1101
Los M, Mozoluk M, Ferrari D, Stepczynska A et al (2002) Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell 13:978–988
Yap E, Tan WL, Ng I, Ng YK (2008) Combinatorial-approached neuroprotection using pan-caspase inhibitor and poly (ADP-ribose) polymerase (PARP) inhibitor following experimental stroke in rats; is there additional benefit? Brain Res 21(1195):130–138
Scovassi AI, Poirier GG (1999) Poly (ADP-ribosylation) and apoptosis. Mol Cell Biochem 199(1):125–137
Benchoua A, Couriaud C, Gueágan C et al (2002) Active caspase-8 translocates into the nucleus of apoptotic cells to inactivate poly (ADP-ribose) polymerase-2. J Biol Chem 277(37):34217–34222
Hoshino J, Koeppel C, Westhäuser E (1994) 3-Aminobenzamide enhances dexamethasone-mediated mouse thymocyte depletion in vivo: implication for a role of poly ADP-ribosylation in the negative selection of immature thymocytes. Biochim Biophys Acta Gen Subj 1201(3):516–522
Yamamoto K, Tsukidate K, John FL (1993) Differing effects of the inhibition of poly (ADP-ribose) polymerase on the course of oxidative cell injury in hepatocytes and fibroblasts. Biochem Pharmacol 46(3):483–491
Wright SC, Kumar P, Tam AW, Shen N, Varma M, Larrick JW (1992) Apoptosis and DNA fragmentation precede TNF-induced cytolysis in U937 cells. J Cell Biochem 48(4):344–355
Kuo ML, Chau YP, Wang JH, Shiah SG (1996) Inhibitors of poly (ADP-ribose) polymerase block nitric oxide-induced apoptosis but not differentiation in human leukemia HL-60 cells. Biochem Biophys Res Commun 219(2):502–508
Bhatia S, Rawal R, Sharma P, Singh T, Singh M, Singh V (2021) Mitochondrial dysfunction in Alzheimer's disease: opportunities for drug development. Curr Neuropharmacol 20(4):675–692
Baxter P, Chen Y, Xu Y, Swanson RA (2014) Mitochondrial dysfunction induced by nuclear poly (ADP-ribose) polymerase-1: a treatable cause of cell death in stroke. Transl Stroke Res 5(1):136–144
Alano CC, Ying W, Swanson RA (2004) Poly (ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J Biol Chem 279(18):18895–18902
Xu Y, Huang S, Liu ZG, Han J (2006) Poly (ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. J Biol Chem 281(13):8788–8795
van Wijk SJ, Hageman GJ (2005) Poly (ADP-ribose) polymerase-1 mediated caspase-independent cell death after ischemia/reperfusion. Free Radic Biol Med 39(1):81–90
Kadam A, Jubin T, Roychowdhury R, Begum R (2020) Role of PARP-1 in mitochondrial homeostasis. Biochim Biophys Acta (BBA) Gen Subj 1864(10):129669
Singh T, Batabyal T, Kapur J (2022) Neuronal circuits sustaining neocortical-injury-induced status epilepticus. Neurobiol Dis 165:105633
Mandir AS, Poitras MF, Berliner AR et al (2000) NMDA but not non-NMDA excitotoxicity is mediated by poly (ADP-ribose) polymerase. J Neurosci 20(21):8005–8011
Gilliams-Francis KL, Quaye AA, Naegele JR (2003) PARP cleavage, DNA fragmentation, and pyknosis during excitotoxin-induced neuronal death. Exp Neurol 184(1):359–372
Cosi C, Suzuki H, Milani D et al (1994) Poly (ADP-ribose) polymerase: early involvement in glutamate-induced neurotoxicity in cultured cerebellar granule cells. J Neurosci Res 39(1):38–46
Meli E, Pangallo M, Baronti R et al (2003) Poly (ADP-ribose) polymerase as a key player in excitotoxicity and post-ischemic brain damage. Toxicol Lett 139(2–3):153–162
Komjáti K, Besson VC, Szabo C (2005) Poly (adp-ribose) polymerase inhibitors as potential therapeutic agents in stroke and neurotrauma. Curr Drug Targets CNS Neurol Disord 4(2):179–194
Kabra DG, Thiyagarajan M, Kaul CL, Sharma SS (2004) Neuroprotective effect of 4-amino-1,8-napthalimide, a poly (ADP ribose) polymerase inhibitor in middle cerebral artery occlusion-induced focal cerebral ischemia in rat. Brain Res Bull 62(5):425–433
Kato N, Morita H, Sugiyama T et al (2000) Evaluation of the poly (ADP-ribose) polymerase gene in human stroke. Atherosclerosis 148(2):345–352
Wallis RA, Panizzon KL, Henry D, Wasterlain CG (1993) Neuroprotection against nitric oxide injury with inhibitors of ADP-ribosylation. NeuroReport 5(3):245–248
Wüllner U, Weller M, Groscurth P et al (1997) Evidence for an active type of cell death with ultrastructural features distinct from apoptosis: the effects of 3-acetylpyridine neurotoxicity. Neuroscience 81(3):721–734
Cookson MR, Ince PG, Shaw PJ (1998) Peroxynitrite and hydrogen peroxide induced cell death in the NSC34 neuroblastoma × spinal cord cell line: role of poly (ADP-ribose) polymerase. J Neurochem 70(2):501–508
Tasker RC, Sahota SK, Cotter FE, Williams SR (1998) Early postischemic dantrolene-induced amelioration of poly (ADP-ribose) polymerase-related bioenergetic failure in neonatal rat brain slices. J Cereb Blood Flow Metab 18(12):1346–1356
Ying W, Swanson RA (2000) The poly (ADP-ribose) glycohydrolase inhibitor gallotannin blocks oxidative astrocyte death. NeuroReport 11(7):1385–1388
Kruman II, Culmsee C, Chan SL et al (2000) Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci 20(18):6920–6926
Oka M, Hirouchi M, Itoh Y, Ukai Y (2000) Involvement of peroxynitrite and hydroxyradical generated from nitric oxide in hypoxia/reoxygenation injury in rat cerebrocortical slices. Neuropharmacology 39(7):1319–1330
Abdelkarim GE, Gertz K, Harms C et al (2001) Protective effects of PJ34, a novel, potent inhibitor of poly (ADP-ribose) polymerase (PARP) in in vitro and in vivo models of stroke. Int J Mol Med 7(3):255–260
Ying W, Sevigny MB, Chen Y, Swanson RA (2001) Poly (ADP-ribose) glycohydrolase mediates oxidative and excitotoxic neuronal death. Proc Natl Acad Sci USA 98(21):12227–12232
Ullrich O, Diestel A, Eyüpoglu IY, Nitsch R (2001) Regulation of microglial expression of integrins by poly (ADP-ribose) polymerase-1. Nat Cell Biol 3:1035–1042
Moroni F, Meli E, Peruginelli F et al (2001) Poly (ADP-ribose) polymerase inhibitors attenuate necrotic but not apoptotic neuronal death in experimental models of cerebral ischemia. Cell Death Differ 8:921–932
Sonee M, Martens JR, Mukherjee SK (2002) Nicotinamide protects HCN2 cells from the free radical generating toxin, tertiary-butylhydroperoxide (t-BuOOH). Neurotoxicity Res 4(7–8):595–599
Sonee M, Martens JR, Evers MR, Mukherjee SK (2003) The effect of tertiary butylhydroperoxide and nicotinamide on human cortical neurons. Neurotoxicol 24(3):443–448
Huang F, Vemuri MC, Schneider JS (2004) Modulation of ATP levels alters the mode of hydrogen peroxide-induced cell death in primary cortical cultures: effects of putative neuroprotective agents. Brain Res 997(1):79–88
Mao J, Price DD, Zhu J, Lu J, Mayer DJ (1997) The inhibition of nitric oxide-activated poly (ADP-ribose) synthetase attenuates transsynaptic alteration of spinal cord dorsal horn neurons and neuropathic pain in the rat. Pain 72(3):355–366
Tokime T, Nozaki K, Sugino T, Kikuchi H, Hashimoto N, Ueda K (1998) Enhanced poly (ADP-ribosyl) ation after focal ischemia in rat brain. J Cereb Blood Flow Metab 18(9):991–997
Lo EH, Bosque-Hamilton P, Meng W (1998) Inhibition of poly(ADP-ribose) polymerase: reduction of ischemic injury and attenuation of N-methyl-D-aspartate-induced neurotransmitter dysregulation. Stroke 9(4):830–836
Takahashi K, Pieper AA, Croul SE, Zhang J, Snyder SH, Greenberg JH (1999) Post-treatment with an inhibitor of poly (ADP-ribose) polymerase attenuates cerebral damage in focal ischemia. Brain Res 829(1–2):46–54
Martin DR, Lewington AJ, Hammerman MR, Padanilam BJ (2000) Inhibition of poly (ADP-ribose) polymerase attenuates ischemic renal injury in rats. Am J Physiol Regul Integr Comp Physiol 279(5):R1834–R1840
Plaschke K, Kopitz J, Weigand MA, Martin E, Bardenheuer HJ (2000) The neuroprotective effect of cerebral poly (ADP-ribose) polymerase inhibition in a rat model of global ischemia. Neurosci Lett 284(1–2):109–112
Ito Z, Serizawa F, Tabuchi K et al (2001) Poly (adenosine diphosphate-ribose) synthetase inhibitor 3-aminobenzamide alleviates cochlear dysfunction induced by transient ischemia. Ann Otol Rhinol Laryngol 110(2):118–121
Satoh M, Date I, Nakajima M et al (2001) Inhibition of poly (ADP-ribose) polymerase attenuates cerebral vasospasm after subarachnoid hemorrhage in rabbits. Stroke 32(1):225–230
Yang J, Klaidman LK, Chang ML et al (2002) Nicotinamide therapy protects against both necrosis and apoptosis in a stroke model. Pharmacol Biochem Behav 73(4):901–910
Goto S, Xue R, Sugo N et al (2002) Poly (ADP-ribose) polymerase impairs early and long-term experimental stroke recovery. Stroke 33(4):1101–1106
Gupta S, Kaul CL, Sharma SS (2004) Neuroprotective effect of combination of poly (ADP-ribose) polymerase inhibitor and antioxidant in middle cerebral artery occlusion induced focal ischemia in rats. Neurol Res 26(1):103–107
Iwashita A, Tojo N, Matsuura S et al (2004) A novel and potent poly (ADP-ribose) polymerase-1 inhibitor, FR247304 (5-chloro-2-[3-(4-phenyl-3, 6-dihydro-1 (2H)-pyridinyl) propyl]-4 (3H)-quinazolinone), attenuates neuronal damage in in vitro and in vivo models of cerebral ischemia. J Pharmacol Exp Ther 310(2):425–436
Hua HT, Albadawi H, Entabi F, Conrad M et al (2005) Polyadenosine diphosphate-ribose polymerase inhibition modulates skeletal muscle injury following ischemia reperfusion. Arch Surg 140:344–351
Kauppinen TM, Suh SW, Berman AE, Hamby AM, Swanson RA (2009) Inhibition of poly (ADP-ribose) polymerase suppresses inflammation and promotes recovery after ischemic injury. J Cereb Blood Flow Metab 29(4):820–829
Klöfers M, Kohaut J, Bendix I et al (2017) Effects of poly (ADP-ribose) polymerase-1 inhibition in a neonatal rodent model of hypoxic-ischemic injury. BioMed Res Int 2017:2924848
Ba X, Garg NJ (2011) Signaling mechanism of poly (ADP-ribose) polymerase-1 (PARP-1) in inflammatory diseases. Am J Pathol 178(3):946–955
Bai P, Nagy L, Fodor T, Liaudet L, Pacher P (2015) Poly (ADP-ribose) polymerases as modulators of mitochondrial activity. Trends Endocrinol Metab 26(2):75–83
Khan H, Singh A, Thapa K, Garg N, Grewal AK, Singh TG (2021) Therapeutic modulation of the phosphatidylinositol 3-kinases (PI3K) pathway in cerebral ischemic injury. Brain Res 1761:147399
Zhu H, Zhang Y, Shi Z et al (2016) The neuroprotection of liraglutide against ischaemia-induced apoptosis through the activation of the PI3K/AKT and MAPK pathways. Sci Rep 6(1):1–1
Gao L, Kwan JC, Macdonald PS, Yang L, Preiss T, Hicks M (2007) Improved poststorage cardiac function by poly (ADP-ribose) polymerase inhibition: role of phosphatidylinositol 3-kinase Akt pathway. Transplantation 84(3):380–386
Li L, Qu Y, Mao M, Xiong Y, Mu D (2008) The involvement of phosphoinositid 3-kinase/Akt pathway in the activation of hypoxia-inducible factor-1α in the developing rat brain after hypoxia–ischemia. Brain Res 1197:152–158
Koh SH, Park Y, Song CW et al (2004) The effect of PARP inhibitor on ischaemic cell death, its related inflammation and survival signals. Eur J Neurosci 20(6):1461–1472
Zhang Z, Yao L, Yang J, Wang Z, Du G (2018) PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia. Mol Med Rep 18(4):3547–3554
Tapodi A, Debreceni B, Hanto K et al (2005) Pivotal role of Akt activation in mitochondrial protection and cell survival by poly (ADP-ribose) polymerase-1 inhibition in oxidative stress. J Biol Chem 280(42):35767–35775
Singh T, Joshi S, Williamson JM, Kapur J (2020) Neocortical injury–induced status epilepticus. Epilepsia 61(12):2811–2824
Tapodi A, Bognar Z, Szabo C, Gallyas F, Sumegi B, Hocsak E (2019) PARP inhibition induces Akt-mediated cytoprotective effects through the formation of a mitochondria-targeted phospho-ATM-NEMO-Akt-mTOR signalosome. Biochem Pharmacol 162:98–108
Prasad SS, Russell M, Nowakowska M (2011) Neuroprotection induced in vitro by ischemic preconditioning and postconditioning: modulation of apoptosis and PI3K–Akt pathways. J Mol Neurosci 43(3):428–442
Li X, Huang Q, Wang M et al (2018) Compound K inhibits autophagy-mediated apoptosis through activation of the PI3K-Akt signaling pathway thus protecting against ischemia/reperfusion injury. Cell Physiol Biochem 47(6):2589–2601
Mester L, Szabo A, Atlasz T et al (2009) Protection against chronic hypoperfusion-induced retinal neurodegeneration by PARP inhibition via activation of PI-3-kinase Akt pathway and suppression of JNK and p38 MAP kinases. Neurotoxicol Res 16(1):68–76
McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV (2005) Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem 280(21):20493–20502
Ashabi G, Khodagholi F, Khalaj L, Goudarzvand M, Nasiri M (2014) Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1α pathway. Metab Brain Dis 29(1):47–58
Virág L, Robaszkiewicz A, Rodriguez-Vargas JM, Oliver FJ (2013) Poly (ADP-ribose) signaling in cell death. Mol Aspects Med 34(6):1153–1167
Huang Q, Lou T, Wang M et al (2020) Compound K inhibits autophagy-mediated apoptosis induced by oxygen and glucose deprivation/reperfusion via regulating AMPK-mTOR pathway in neurons. Life Sci 254:117793
Thapa K, Khan H, Sharma U, Grewal AK, Singh TG (2020) Poly (ADP-ribose) polymerase-1 as a promising drug target for neurodegenerative diseases. Life Sci 267:118975
Zhao B, Qiang L, Joseph J, Kalyanaraman B, Viollet B, He YY (2016) Mitochondrial dysfunction activates the AMPK signaling and autophagy to promote cell survival. Genes Dis 3(1):82–87
Kalra P, Khan H, Kaur A, Singh TG (2022) Mechanistic insight on autophagy modulated molecular pathways in cerebral ischemic injury: from preclinical to clinical perspective. Neurochem Res 47(4):825–843
Hagberg H, Wilson MA, Matsushita H et al (2004) PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem 90(5):1068–1075
McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD (2005) Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab 25(4):502–512
Bressenot A, Marchal S, Bezdetnaya L, Garrier J, Guillemin F, Plénat F (2009) Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma. J Histochem Cytochem 57(4):289–300
Boulares AH, Yakovlev AG, Ivanova V et al (1999) Role of poly (ADP-ribose) polymerase (PARP) cleavage in apoptosis: caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem 274(33):22932–22940
Sairanen T, Szepesi R, Karjalainen-Lindsberg ML, Saksi J, Paetau A, Lindsberg PJ (2009) Neuronal caspase-3 and PARP-1 correlate differentially with apoptosis and necrosis in ischemic human stroke. Acta Neuropathol 118(4):541–552
Shakibaei M, John T, Seifarth C, Mobasheri AL (2007) Resveratrol inhibits IL-1β–induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann NY Acad Sci 1095(1):554–563
Rihal V, Khan H, Kaur A, Singh TG (2022) Vitamin D as therapeutic modulator in cerebrovascular diseases: a mechanistic perspectives. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2022.2050349
Ferrer I, Planas AM (2003) Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol 62(4):329–339
Germain M, Affar EB, D’Amours D, Dixit VM, Salvesen GS, Poirier GG (1999) Cleavage of automodified poly (ADP-ribose) polymerase during apoptosis: evidence for involvement of caspase-7. J Biol Chem 274(40):28379–28384
Saklani P, Khan H, Gupta S, Kaur A, Singh TG (2022) Neuropeptides: potential neuroprotective agents in ischemic injury. Life Sci 288:120186
Prabhakaran K, Li L, Borowitz JL, Isom GE (2004) Caspase inhibition switches the mode of cell death induced by cyanide by enhancing reactive oxygen species generation and PARP-1 activation. Toxicol Appl Pharmacol 195(2):194–202
Zhang N, Chen Y, Jiang R et al (2011) PARP and RIP 1 are required for autophagy induced by 11’-deoxyverticillin A, which precedes caspase-dependent apoptosis. Autophagy 7(6):598–612
Krupinski J, Ferrer I, Barrachina M, Secades JJ, Mercadal J, Lozano R (2002) CDP-choline reduces pro-caspase and cleaved caspase-3 expression, nuclear DNA fragmentation, and specific PARP-cleaved products of caspase activation following middle cerebral artery occlusion in the rat. Neuropharmacology 42(6):846–854
Laudisi F, Sambucci M, Pioli C (2011) Poly (ADP-ribose) polymerase-1 (PARP-1) as immune regulator. Endocr Metab Immune Disord Drug Targets 11(4):326–333
Strosznajder RP, Czubowicz K, Jesko H, Strosznajder JB (2010) Poly (ADP-ribose) metabolism in brain and its role in ischemia pathology. Mol Neurobiol 41(2):187–196
Hamby AM, Suh SW, Kauppinen TM, Swanson RA (2007) Use of a poly (ADP-ribose) polymerase inhibitor to suppress inflammation and neuronal death after cerebral ischemia-reperfusion. Stroke 38(2):632–636
Geraets L, Moonen HJ, Brauers K, Gottschalk RW, Wouters EF, Bast A, Hageman GJ (2007) Flavone as PARP-1 inhibitor: its effect on lipopolysaccharide induced gene-expression. Eur J Pharmacol 573(1–3):241–248
Castri P, Lee YJ, Ponzio T et al (2014) Poly (ADP-ribose) polymerase-1 and its cleavage products differentially modulate cellular protection through NF-kB-dependent signaling Biochim Biophys Acta (BBA) Mol Cell Res 1843(3):640–651
Wang G, Huang X, Li Y, Guo K, Ning P, Zhang Y (2013) PARP-1 inhibitor, DPQ, attenuates LPS-induced acute lung injury through inhibiting NF-κB-mediated inflammatory response. PLoS ONE 8(11):e79757
Martire S, Fuso A, Rotili D et al (2013) PARP-1 modulates amyloid beta peptide-induced neuronal damage. PLoS ONE 8(9):e72169
Spina-Purrello V, Patti D, Giuffrida-Stella AM, Nicoletti VG (2008) PARP and cell death or protection in rat primary astroglial cell cultures under LPS/IFNγ induced proinflammatory conditions. Neurochem Res 33(12):2583–2592
Koh SH, Chang DI, Kim HT et al (2005) Effect of 3-aminobenzamide, PARP inhibitor, on matrix metalloproteinase-9 level in plasma and brain of ischemic stroke model. Toxicology 214(1–2):131–139
Gao Y, Bai L, Zhou W et al (2020) PARP-1-regulated TNF-α expression in the dorsal root ganglia and spinal dorsal horn contributes to the pathogenesis of neuropathic pain in rats. Brain Behav Immun 88:482–496
Huang D, Yang CZ, Yao L, Wang Y, Liao YH, Huang K (2008) Activation and overexpression of PARP-1 in circulating mononuclear cells promote TNF-α and IL-6 expression in patients with unstable angina. Arch Med Res 39(8):775–784
Esposito E, Cuzzocrea S (2009) TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr Med Chem 16(24):3152–3167
Sosna J, Voigt S, Mathieu S et al (2014) TNF-induced necroptosis and PARP-1-mediated necrosis represent distinct routes to programmed necrotic cell death. Cell Mol Life Sci 71(2):331–348
Drel VR, Lupachyk S, Shevalye H et al (2010) New therapeutic and biomarker discovery for peripheral diabetic neuropathy: PARP inhibitor, nitrotyrosine, and tumor necrosis factor-α. Endocrinology 151(6):2547–2555
Haddad M, Rhinn H, Bloquel C et al (2006) Anti-inflammatory effects of PJ34, a poly (ADP-ribose) polymerase inhibitor, in transient focal cerebral ischemia in mice. Br J Pharmacol 149(1):23–30
Ismael S, Zhao L, Nasoohi S, Ishrat T (2018) Inhibition of the NLRP3-inflammasome as a potential approach for neuroprotection after stroke. Sci Rep 8(1):1–9
Rom S, Zuluaga-Ramirez V, Reichenbach NL et al (2016) PARP inhibition in leukocytes diminishes inflammation via effects on integrins/cytoskeleton and protects the blood-brain barrier. J Neuroinflammation 13(1):1–6
Haile WB, Echeverry R, Wu F, Guzman J, An J, Wu J, Yepes M (2010) Tumor necrosis factor-like weak inducer of apoptosis and fibroblast growth factor-inducible 14 mediate cerebral ischemia-induced poly (ADP-ribose) polymerase-1 activation and neuronal death. Neuroscience 171(4):1256–1264
Węsierska-Gądek J, Wojciechowski J, Schmid G (2003) Phosphorylation regulates the interaction and complex formation between wt p53 protein and PARP-1. J Cell Biochem 89(6):1260–1348
Gupta A, Shah K, Oza MJ, Behl T (2019) Reactivation of p53 gene by MDM2 inhibitors: A novel therapy for cancer treatment. Biomed Pharmacother 109:484–492
Elkholi R, Chipuk JE (2014) How do I kill thee? Let me count the ways: p53 regulates PARP-1 dependent necrosis. BioEssays 36(1):46–51
Valenzuela MT, Guerrero R, Núñez MI et al (2002) PARP-1 modifies the effectiveness of p53-mediated DNA damage response. Oncogene 21(7):1108–1116
Okuda A, Kurokawa S, Takehashi M et al (2017) Poly (ADP-ribose) polymerase inhibitors activate the p53 signaling pathway in neural stem/progenitor cells. BMC Neurosci 18(1):14
Leak RK, Zhang L, Luo Y et al (2013) Peroxiredoxin 2 battles poly (ADP-ribose) polymerase 1-and p53-dependent prodeath pathways after ischemic injury. Stroke 44(4):1124–1134
Khan H, Tiwari P, Kaur A, Singh TG (2021) Sirtuin acetylation and deacetylation: a complex paradigm in neurodegenerative disease. Mol Neurobiol 58(8):3903–3917
Kolthur-Seetharam U, Dantzer F, McBurney MW, Murcia GD, Sassone-Corsi P (2006) Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle 5(8):873–877
Koronowski KB, Perez-Pinzon MA (2015) Sirt1 in cerebral ischemia. Brain Circ 1(1):69
Zhang JF, Zhang YL, Wu YC (2018) The role of Sirt1 in ischemic stroke: pathogenesis and therapeutic strategies. Front Neurosci 12:833
Deng H, Mi MT (2016) Resveratrol attenuates Aβ 25–35 caused neurotoxicity by inducing autophagy through the TYrRS-PARP1-SIRT1 signaling pathway. Neurochem Res 41(9):2367–2379
Bandopadhyay R, Singh T, Ghoneim MM, Alshehri S, Angelopoulou E, Paudel YN, Piperi C, Ahmad J, Alhakamy NA, Alfaleh MA, Mishra A (2021) Recent developments in diagnosis of epilepsy: scope of microRNA and technological advancements. Biology 10(11):1097
Mao Z, Hine C, Tian X et al (2011) SIRT6 promotes DNA repair under stress by activating PARP1. Science 332(6036):1443–1446
Bai P, Cantó C, Oudart H et al (2011) PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13(4):461–468
Hekmatimoghaddam S, Firoozabadi AD, Zare-Khormizi MR, Pourrajab F (2017) Sirt1 and Parp1 as epigenome safeguards and microRNAs as SASP-associated signals, in cellular senescence and aging. Ageing Res Rev 40:120–141
Cohen-Armon M (2007) PARP-1 activation in the ERK signaling pathway. Trends Pharmacol Sci 28(11):556–560
Cohen-Armon M, Visochek L, Rozensal D et al (2007) DNA-independent PARP-1 activation by phosphorylated ERK2 increases Elk1 activity: a link to histone acetylation. Mol Cell 25(2):297–308
Liu L, Ke Y, Jiang X et al (2012) Lipopolysaccharide activates ERK–PARP-1–RelA pathway and promotes nuclear factor–κB transcription in murine macrophages Hum Immunol 73(5):439–447
Ethier C, Labelle Y, Poirier GG (2007) PARP-1-induced cell death through inhibition of the MEK/ERK pathway in MNNG-treated HeLa cells. Apoptosis 12(11):2037–2049
Kauppinen TM, Chan WY, Suh SW, Wiggins AK, Huang EJ, Swanson RA (2006) Direct phosphorylation and regulation of poly (ADP-ribose) polymerase-1 by extracellular signal-regulated kinases 1/2. Proc Natl Acad Sci USA 103(18):7136–7141
Visochek L, Grigoryan G, Kalal A et al (2016) A PARP1-ERK2 synergism is required for the induction of LTP. Sci Rep 6(1):1–6
Domercq M, Mato S, Soria FN, Sánchez-gómez MV, Alberdi E, Matute C (2013) Zn2+-induced ERK activation mediates PARP-1-dependent ischemic-reoxygenation damage to oligodendrocytes. Glia 61(3):383–393
López-Neblina F, Toledo-Pereyra LH (2006) Phosphoregulation of signal transduction pathways in ischemia and reperfusion. J Surg Res 134(2):292–299
Sugawara T, Fujimura M, Noshita N et al (2004) Neuronal death/survival signaling pathways in cerebral ischemia. Neurochem Res 1(1):17–25
Mullonkal CJ, Toledo-Pereyra LH (2007) Akt in ischemia and reperfusion. J Investig Surg 20(3):195–203
Acknowledgements
The authors are grateful to the Chitkara College of Pharmacy, Chitkara University, Rajpura, Patiala, Punjab, India, for providing the necessary facilities to carry out the research work.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
Conceptualization: Conceived and designed the experiments: TGS. Analyzed the data: HK, TGS. Wrote the manuscript: PTHK. Editing of the manuscript: AKG. Critically reviewed the article: TGS.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors read and given their consent for the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tiwari, P., Khan, H., Singh, T.G. et al. Poly (ADP-ribose) polymerase: An Overview of Mechanistic Approaches and Therapeutic Opportunities in the Management of Stroke. Neurochem Res 47, 1830–1852 (2022). https://doi.org/10.1007/s11064-022-03595-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03595-z