Abstract
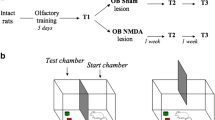
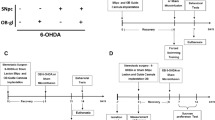
There is increasing preclinical and clinical data supporting a potential association between Traumatic Brain Injury (TBI) and Parkinson’s disease (PD). It has been suggested that the glutamate-induced excitotoxicity underlying TBI secondary neuronal degeneration (SND) might be associated with further development of PD. Interestingly, an accumulation of extracellular glutamate and olfactory dysfunction are both sharing pathological conditions in TBI and PD. The possible involvement of glutamate excitotoxicity in olfactory dysfunction has been recently described, however, the role of olfactory bulbs (OB) glutamate excitotoxicity as a possible mechanism involved in the association between TBI and PD-related neurodegeneration has not been investigated yet. We examined the number of nigral dopaminergic neurons (TH +), nigral α-synuclein expression, the striatal dopamine transporter (DAT) expression, and motor performance after bilateral OB N-Methyl-D-Aspartate (NMDA)-induced excitotoxic lesions in rodents. Bulbar NMDA administration induced a decrease in the number of correct choices in the discrimination tests one week after lesions (p < 0.01) and a significant decrease in the number of nigral DAergic neurons (p < 0.01) associated with an increase in α-synuclein expression (p < 0.01). No significant striatal changes in DAT expression or motor alterations were observed. Our results show an association between TBI-induced SND and PD-related neurodegeneration suggesting that the OB excitotoxicity occurring in TBI SND may be a filling gap mechanism underlying the link between TBI and PD-like pathology.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Van Bregt DR, Thomas TC, Hinzman JM et al (2012) Substantia nigra vulnerability after a single moderate diffuse brain injury in the rat. Exp Neurol 234:8–19
Shimada R, Abe K, Furutani R, Kibayashi K (2014) Changes in dopamine transporter expression in the midbrain following traumatic brain injury: an immunohistochemical and in situ hybridization study in a mouse model. Neurol Res 36:239–246
Acosta SA, Tajiri N De la Pena I, Bastawrous M, Sanberg PR, Kaneko Y, Borlongan CV (2015) Alpha-synuclein as a pathological link between chronic traumatic Brain injury and Parkinson’s disease. J Cell Physiol 230:1024-1032
Taylor KM, Saint-Hilaire MH, Sudarsky L et al (2016) Head injury at early ages is associated with risk of Parkinson’s disease. Parkinsonism Relat Disord 23:57–61
Chen YH, Huang EY, Kuo TT, Miller J, Chiang TH, Hoffer BF (2017) Impact of traumatic brain injury on dopaminergic transmission. Cell Transplant 26:1156–1158
Cruz-Haces M, Tang J, Acosta G, Fernandez J, Shi R (2017) Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases. Transl Neurodeg 6:20
Delic V, Beck KD, Pang KCH, Citron BA (2020) Biological links between traumatic brain injury and Parkinson’s disease. Acta Neuropathol Comm 8:45
Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA (2003) Head trauma preceding PD: a case-control study. Neurology 60:1610–1615
Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW (2006) Head injury and Parkinson’s disease risk in twins. Ann Neurol 60:65–72
Jafari S, Etminan M, Aminzadeh F, Samii A (2013) A head injury and risk of Parkinson’s disease: a systematic review and meta-analysis. Mov Dis 28:1222–1229
Gardner RC, Burke JF, Nettiksimmons J et al (2015) Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol 77:987–995
Gardner RC, Al B, Barnes DE, Li Y, Boscardin J, Yaffe K (2018) Mild TBI and risk of Parkinson disease: A chronic effect of neurotrauma consortium study. Neurology 90:e1771–e1779
Spillantini MG, Schmidt ML, Lee VM, Tojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840
Schapira AHV, Chaudhuri KR, Jenner P (2017) Non-motor features of Parkinson’s disease. Nat Rev Neurosci 18:435–450
Bloem BR, Okun MS, Klein C (2021) Parkinson’s disease. Lancet 397:2284–2303
Jenkins PO, De Simoni S, Bourke NJ et al (2018) Dopaminergic abnormalities following traumatic brain injury. Brain 141:797–810
Jenkins PO, Roussakis AA, De Simoni S, Bourke N, Fleminger J, Cole J, Piccini P, Sharp D (2020) Distinct dopaminergic abnormalities in traumatic brain injury and Parkinson’s disease. J Neurol Neurosurg Psychiatry 91:631–637
Womack KB, Dubiel R, Callender L et al (2020) 123I-Iofluopane single-photon emission computed tomography as an imaging biomarker of pre-synaptic dopaminergic system after moderate-to-severe traumatic brain injury. J Neurotrauma 37:2113–2119
Donnemiller E, Brenneis C, Wissel J et al (2000) Impaired dopaminergic neurotransmission in patients with traumatic brain injury: a SPECT study using 123I–β-CIT and 123I-IBZM. Eur J Nucl Med 27:1410–1414
Haugen J, Müller ML, Kotagal V et al (2016) Prevalence of impaired odor identification in Parkinson disease with imaging evidence of nigrostriatal denervation. J Neural Transm 123:421–424
Marin C, Vilas D, Langdon C, Alobid I, López-Chacón M, Haehner A, Hummel T, Mullol J (2018) Olfactory dysfunction in neurodegeneration diseases. Curr Allergy Asthma Rep 18:42
Schofield PW, Moore TM, Gardner A (2014) Traumatic brain injury and olfaction: a systematic review. Front Neurol 5:5
Marin C, Langdon C, Alobid I, Mullol J (2020) Olfactory dysfunction in traumatic brain injury: role of neurogenesis. Curr Allergy Asthma Rep 20:55
Bae YH, Joo H, Bae J et al (2018) Brain injury induces HIF-1-alpha-dependent transcriptional activation of LRRK2 that exacerbates brain damage. Cell Death Dis 9:1125
Selvakumar GP, Ahmed ME, Iyer SS et al (2020) Absence of glia maturation factor protects from axonal injury and motor behavioral impairments after traumatic brain injury. Exp Neurobiol 29:230–248
Marin C, Laxe S, Langdon C, Berenguer J, Lehrer E, Mariño-Sánchez F, Alobid I, Bernabeu M, Mullol J (2017) Olfactory function in an excitotoxic model for secondary neuronal degeneration: Role of dopaminergic interneurons. Neuroscience 364:28–44
Marin C, Laxe S, Langdon C, Alobid I, Berenguer J, Fuentes M, Bernabeu M, Mullol J (2019) Olfactory training prevents olfactory dysfunction induced by bulbar excitotoxic lesions: role of neurogenesis and dopaminergic interneurons. Mol Neurobiol 56:8063–8075
Marin C, Langdon C, Alobid I, Fuentes M, Bonastre M, Mullol J (2019) Recovery of olfactory function after excitotoxic lesion of the olfactory bulbs is associated with increases in bulbar SIRT1 and SIRT4 expressions. Mol Neurobiol 56:5643–5653
Ladak AA, Enam SA, Ibrahim M (2019) A review of the molecular mechanisms of traumatic brain injury. World Neurosurg 131:126–132
Jarrahi A, Brain M, Ahluwalia M et al (2020) Revisiting traumatic brain injury: from molecular mechanisms to therapeutic interventions. Biomedicines 8:389
Thapa K, Khan H, Singh TG, Kaur A (2021) Traumatic brain injury: mechanistic insight on pathophysiology and potential therapeutic targets. J Mol Neurosci 71:1725–1742
Acosta G, Race N, Herr S, Fernandez J, Tang J, Rogers E, Shi R (2019) Acrolein-mediated alpha-synuclein pathology involvement in the early post-injury pathogenesis of mild blast-induced parkinsonian neurodegeneration. Mol Cell Neurosci 98:140–154
Ng SY, Lee AYW (2019) Traumatic brain injuries: Pathophysiology and potential therapeutic targets. Front Cell Neurosci 13:528
Bertogliat MJ, Morris-Blanco KC, Vemuganti R (2020) Epigenetic mechanisms of neurodegenerative diseases and acute brain injury. Neurochem Int 133:104642
Faden AI, Dememdiuk P, Panter SS, Vink R (1989) The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 244:798–800
Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL (2013) Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur Pharmacol 698:6–18
Chamoun R, Suki D, Gopinath SP, Goodman JC, Robertson C (2010) Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J Neurosurg 113:564–570
Abramov AY, Duchen MR (2008) Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim Biophys Acta 1777:953–964
Faden AI, O’Leary DM, Fan L, Bao W, Mullins PG, Movsesyan VA (2001) Selective blockade of the mGLuR1 receptor reduces traumatic neuronal injury in vitro and improves outcome after brain trauma. Exp Neurol 167:435–444
Rao VL, Dogan A, Todd KG, Bowen KK, Dempsey RJ (2001) Neuroprotection by memantine, a non-competitive NMDA receptor antagonist after traumatic brain injury in rats. Brain Res 911:96–100
Iovino L, Tremblay ME, Civiero L (2020) Glutamate induced excitotoxicity in Parkinson’s disease: The role of glial cells. J Pharmacol Sci 144:151–164
Wang J, Wang F, Mai D, Qu S (2020) Molecular mechanisms of glutamate toxicity in Parkinson’s disease. Front Neurosci 14:585584
Wu X, Meng X, Tan F, Jiao Z, Zhang X, Tong H, He X, Luo X, Xu P, Qu S (2019) Regulatory mechanisms of miR-543-3p `n GLT-1 in a mouse model of Parkinson’s disease. ACS Chem Neurosci 10:1791–1800
Pajarillo E, Rizor A, Lee J, Aschner M, Lee W (2019) The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: potential targets for neurotherapeutics. Neuropharmacology 161:107559
Zhang Y, Meng X, Jiao Z, Liu Y, Zhang X, Qu S (2019) Generation of a novel model of Parkinson’s disease via targeted knockdown of glutamate transporter GLT-1 in the substantia nigra. ACS Chem Neurosci 11:406–411
Hinzman JM, Wilson JA, Mazeo AT, Bullock MR, Hartings JA (2016) Excitotoxicity and metabolic crisis are associated with spreading depolarizations in severe traumatic brain injury patients. J Neurotrauma 33:1775–1783
Dorsett CR, McGuire JL, DePasquale EA, Gardner AE, Floyd CL, McCullusmith RE (2017) Glutamate neurotransmission in rodent models of traumatic brain injury. J Neurotrauma 34:263–272
Lethbridge R, Hou Q, Harley CW, Yuan Q (2012) Olfactory bulb glomerular NMDA receptors mediate olfactory nerve potentiation and odor preference learning in the neonate rat. PLoS One 7:e35024
Tatti R, Bhaukaurally K, Gschwend O, Seal RP, Edwards RH, Rodriguez I, Carleton A (2014) A population of glomerular glutamatergic neurons controls sensory information transfer in the mouse olfactory bulb. Nat Commun 5:3791
Lee JH, Wei L, Deveau TC, Gu X, Yu SP (2016) Expression of the NMDA receptor subunit GluN3A (NR3A) in the olfactory system and its regulatory role on olfaction in the adult Mouse. Brain Struct Funct 221:3259–3273
Litim N, Morissette M, Di Paolo T (2017) Metabotropic glutamate receptors as therapeutic targets in Parkinson’s disease: An update from the last 5 years of research. Neuropharmacology 115:166–179
Ardiles Y, de la Puente R, Toledo R, Isgor C, Guthrie K (2007) Response of olfactory axons to loss of synaptic targets in the adult mouse. Exp Neurol 207:275–288
Liu H, Guthrie KM (2011) Neuronal replacement in the injured olfactory bulb. Exp Neurol 228:270–282
Mandairon N, Peace S, Karnow A, Kim J, Ennis M, Linster C (2008) Noradrenergic modulation in the olfactory bulb influences spontaneous and reward-motivated discrimination, but not the formation of habituation memory. Eur J Neurosci 27:1210–1219
Escanilla O, Yuhas C, Marzan D, Linster C (2009) Dopaminergic modulation of olfactory bulb processing affects odor discrimination learning in rats. Behav Neurosci 123:828–833
Escanilla O, Arrellanos A, Karnow A, Ennis M, Linster C (2010) Noradrenergic modulation of behavioral odor detection and discrimination thresholds in the olfactory bulb. Eur J Neurosci 32:458–468
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, New York
Mandairon N, Sacquet J, Garcia S, Ravel N, Jourdan F, Didier A (2006) Neurogenic correlates of an olfactory discrimination task in the adult olfactory bulb. Eur J Neurosci 24:3578–3588
Brushdfield AM, Luu T, Callahan B, Giblert PE (2008) A comparison of discrimination and reversal learning for olfactory and visual stimuli in aged rats. Behav Neurosci 122:54–62
Pan YW, Kuo CT, Storm DR, Xia Z (2012) Inducible and targeted deletion of the ERK5 MAP kinase in adult neurogenic regions impairs adult neurogenesis in the olfactory bulb and several forms of olfactory behaviour. PLoS One 7:e49622
Zou J, Pan YW, Wang Z, Chang SY, Wang W, Wang X, Tournier C, Storm DR, Xia Z (2012) Targeted deletion of ERK5 MAP kinase in the developing nervous system impairs development of GABAergic interneurons in the main olfactory bulb and behavioural discrimination between structurally similar odorants. J Neurosci 32:4118–4132
Wang W, Lu S, Li T, Pan YW, Zou J, Abel GM, Xu L, Storm DR, Xia Z (2015) Inducible activation of ERK5 MAP kinase enhances adult neurogenesis in the olfactory bulb and improves olfactory function. J Neurosci 35:7833–7849
Pavlis M, Feretti C, Levy A, Gupta N, Linster C (2006) L-Dopa improves odor discrimination learning in rats. Physiol Behav 87:109–113
Marin C, Bonastre M, Mengod G, Cortés R, Giralt A, Obeso JA, Schapira AH (2014) Early-L-Dopa, but not pramipexole, restores basal ganglia activity in partially 6-OHDA-lesioned rats. Neurobiol Dis 64:36–47
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39:777–787
Khanlou N, Moore DJ, Chana G, Cherner M, Lazzaretto D, Dawes S, Grant I, Masliah E, Everall IP (2009) Increased frequency of α-synuclein in the substantia nigra in HIV infection. J Neurovirol 15:131–138
Impellizzeri D, Campolo M, Bruschetta G, Crupi R, Cordaro M, Paterniti I, Cuzzocrea S, Esposito E (2016) Traumatic Brain injury leads to development of Parkinson’s disease related pathology in mice. Front Neurosci 10:458
Höglingler GU, Alvarez-Fischer D, Arias-Carrion O et al (2015) A new dopaminergic nigro-olfactory projection. Acta Neuropathol 130:333–348
Aydin MD, Kanat A, Hacimuftuoglu A, Ozmen S, Ahiskalioglu A, Kocak MN (2021) A new experimental evidence that olfactory bulb lesion may be a causative factor for substantia nigra degeneration; preliminary study. Int J Neurosci 131:220–227
Lan YL, Li S, Lou JC, Ma XC, Zhang B (2019) The potential roles of dopamine in traumatic brain injury: a preclinical and clinical update. Am J Transl Res 11:2616–2631
Uryu K, Giasson BI, Longhi L et al (2003) Age-dependent synuclein pathology following traumatic brain injury in mice. Exp Neurol 184:214–224
Hutson CB, Mortazavi LCR, F, Giza CC, Hovda D, Chesselet MF, (2011) Traumatic brain injury in adult rats causes progressive nigrostriatal dopaminergic cell loss and enhanced vulnerability to the pesticide paraquat. J Neurotrauma 28:1783–1801
Wagner AK, Sokoloski JE, Ren D et al (2005) Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J Neurochem 95:457–465
Hicks R, Soares H, Smith D, Mcintosh T (1996) Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol 91:236–246
Mallah K, Quanico J, Raffo-Romero A, Cardo T, Aboulouard S, Devos D, Kobeissy F, Zibara K, Salzet M, Fournier I (2019) Mapping spatiotemporal microproteomics landscape in experimental model of traumatic brain injury unveils a link to Parkinson’s disease. Mol Cell Prot 18:1669–1682
Rudow G, O’Brien R, Savonenko AV, Resnick SM, Zonderman AB, Letnikova I, Marsh K, Dawson TM, Crain BJ, West MJ, Troncoso J (2008) Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol 115:461–470
Cheng HC, Ulane CM, Burke RE (2010) Clinical progression in Parkinson’s disease and the neurobiology of axons. Ann Neurol 67:715–725
Marsden CD (1990) Parkinson’s disease. Lancet 335:948–952
Ross GW, Petrovitch H, Abbot RD, Nelson J, Markesbery W, Davis D, Hardman J, Launer L, Masaki K, Tanner CM, White LR (2004) Parkinsonian signs and substantia nigra neuron density in descendents elders without PD. Ann Neurol 56:532–539
Braak H, Del Tredici K, Rüb U, de Vos RAI, Steur ENHJ, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Dis 24:197–211
Uryu K, Chen XH, Martinez D et al (2007) Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in brains. Exp Neurol 208:185–192
Newell KL, Boyer P, Gomez-Tortosa E, Hobbs W, Hedley-Whyte ET, Vonsattel JP, Hyman BT (1999) Alpha-synuclein immunoreactivity is present in axonal swellings in neuroaxonal dystrophy and acute traumatic brain injury. J Neuropathol Exp Neurol 58:1263–1268
Wong J, Hazrati LN (2013) Parkinson’s disease, parkinsonism, and traumatic brain injury. Crit Rev Clin Lab Sci 50:103–106
Mondello S, Buki A, Italiano D, Jeromin A (2013) Alpha-synuclein in CSF of patients with severe traumatic brain injury. Neurology 80:1662–1668
Sheng L, Stewart T, Yang D et al (2020) Erythrocytic α-synuclein acontained in microvesicles regulates astrocytic glutamate homeostasis: a new perspective on Parkinson’s disease pathogenesis. Acta Neuropathol Comm 8:102
Langdon C, Lehrer E, Berenguer J, Laxe S, Alobid I, Ll Q, Mariño-Sánchez F, Bernabeu M, Marin C, Mullol J (2018) Olfactory training in post-traumatic smell impairment: mild improvement in threshold performances: results from a randomized controlled trial. J Neurotrauma 35:2641–2652
Vilas D, Tolosa E, Quintana M, Pont-Sunyer C, Santos M, Casellas A, Valldeoriola F, Compta Y, Martí MJ, Mullol J (2020) Olfaction in LRRK2 linked Parkinson’s disease: is it different from idiopathic Parkinson’s disease. J Parkinsons Dis 10:951–958
Langdon C, Alobid I, Ll Q, Valero A, Picado C, Marin C, Mullol J (2019) Self-perception of olfactory dysfunction is associated with history of traumatic brain injury: post-hoc analysis from the OLFACT survey. Rhinology 57:460–468
Ciofalo A, De Vincentii M, Iannella G, Zambetti G, Giacomelli P, Altissimi G, Greco A, Fuscini M, Pasquariello B, Magliulo G (2018) Mild traumatic brain injury: evaluation of olfactory dysfunction and clinical-neurological characteristics. Brain Inj 32:550–556
Funding
This work was partially supported by a research grant (110610) from Fundació La Marató TV3 and a grant from the Spanish Ministerio de Ciencia, Innovación y Universidades (PI19/00806).
Author information
Authors and Affiliations
Contributions
CM designed the project, performed the behavioral experiments, supervised the experiments, analyzed the data and wrote the manuscript; MF performed the laboratory experiments and technical assistance; IA, VT, MJR-L. performed partial data analysis and revised the manuscript; JM designed the project, supervised the experiments, performed partial data analysis, and revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors have no financial or proprietary interests in any material discussed in this article.
Ethical approval
All experiments were carried out following the European (2010/63/UE) and Spanish (RD 53/2013) regulation for the care and use of laboratory animals and approved by the local Government (Generalitat de Catalunya, #372/17, #373/17). The Ethics Committee from the Hospital Clinic de Barcelona approved this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marin, C., Fuentes, M., Alobid, I. et al. Olfactory Bulb Excitotoxicity as a Gap-Filling Mechanism Underlying the Link Between Traumatic Brain Injury-Induced Secondary Neuronal Degeneration and Parkinson’s Disease-Like Pathology. Neurochem Res 47, 1025–1036 (2022). https://doi.org/10.1007/s11064-021-03503-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03503-x