Abstract
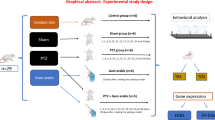
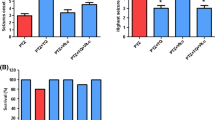
Gamma-decanolactone (GD) has been shown to reduce epileptic behavior in different models, inflammatory decreasing, oxidative stress, and genotoxic parameters. This study assessed the GD effect on the pentylenetetrazole (PTZ) model after acute and subchronic treatment. We evaluated the expression of the inflammatory marker cyclooxygenase-2 (COX-2), GluN2B, a subunit of the NMDA glutamate receptor, adenosine A1 receptor, and GD genotoxicity and mutagenicity. Male and female mice were treated with GD (300 mg/kg) for 12 days. On the tenth day, they were tested in the Hot Plate test. On the thirteenth day, all animals received PTZ (90 mg/kg), and epileptic behavior PTZ-induced was observed for 30 min. Pregabalin (PGB) (30 mg/kg) was used as a positive control. Samples of the hippocampus and blood were collected for Western Blotting analyses and Comet Assay and bone marrow to the Micronucleus test. Only the acute treatment of GD reduced the seizure occurrence and increased the latency to the first stage 3 seizures. Males treated with GD for 12 days demonstrated a significant increase in the expression of the GluN2B receptor and a decrease in the COX-2 expression. Acute and subchronic treatment with GD and PGB reduced the DNA damage produced by PTZ in males and females. There is no increase in the micronucleus frequency in bone marrow after subchronic treatment. This study suggests that GD, after 12 days, could not reduce PTZ-induced seizures, but it has been shown to protect against DNA damage, reduce COX-2 and increase GluN2B expression.












Similar content being viewed by others
Data Availability
The datasets generated during or analyzed during the current study are available from the corresponding author on reasonable request.
References
World Health Organization (2019) Epilepsy. https://www.who.int/health-topics/epilepsy. Accessed 30 July 2020
Devinsky O, Vezzani A, O’Brien TJ et al (2018) Epilepsy. Nat Rev Dis Prim. https://doi.org/10.1038/nrdp.2018.24
Fisher RS, Acevedo C, Arzimanoglou A et al (2014) ILAE official report: a practical clinical definition of epilepsy. Epilepsia 55:475–482. https://doi.org/10.1111/epi.12550
Shimada T, Takemiya T, Sugiura H, Yamagata K (2014) Role of inflammatory mediators in the pathogenesis of epilepsy. Mediat Inflamm. https://doi.org/10.1155/2014/901902
Xanthos DN, Sandkühler J (2014) Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci 15:43–53. https://doi.org/10.1038/nrn3617
Vezzani A, Balosso S, Ravizza T (2019) Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol 15:459–472. https://doi.org/10.1038/s41582-019-0217-x
Temp FR, Marafiga JR, Milanesi LH et al (2017) Cyclooxygenase-2 inhibitors differentially attenuate pentylenetetrazol-induced seizures and increase of pro- and anti-inflammatory cytokine levels in the cerebral cortex and hippocampus of mice. Eur J Pharmacol 810:15–25. https://doi.org/10.1016/j.ejphar.2017.05.013
Rawat C, Kukal S, Dahiya UR, Kukreti R (2019) Cyclooxygenase-2 (COX-2) inhibitors: future therapeutic strategies for epilepsy management. J Neuroinflammation 16:197. https://doi.org/10.1186/s12974-019-1592-3
Dhir A (2019) An update of cyclooxygenase (COX)-inhibitors in epilepsy disorders. Expert Opin Investig Drugs 28:191–205. https://doi.org/10.1080/13543784.2019.1557147
Akula KK, Dhir A, Kulkarni SK (2008) Rofecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor increases pentylenetetrazol seizure threshold in mice: Possible involvement of adenosinergic mechanism. Epilepsy Res. https://doi.org/10.1016/j.eplepsyres.2007.10.008
Dhir A, Naidu PS, Kulkarni SK (2007) Neuroprotective effect of nimesulide, a preferential COX-2 inhibitor, against pentylenetetrazol (PTZ)-induced chemical kindling and associated biochemical parameters in mice. Seizure. https://doi.org/10.1016/j.seizure.2007.05.016
Katyal J, Kumar H, Gupta YK (2015) Anticonvulsant activity of the cyclooxygenase-2 (COX-2) inhibitor etoricoxib in pentylenetetrazole-kindled rats is associated with memory impairment. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2014.12.032
Guzzo FE, Rodrigues MK et al (2018) Effect of dexamethasone on seizures and inflammatory profile induced by kindling seizure model. J Neuroimmunol 325:92–98. https://doi.org/10.1016/j.jneuroim.2018.10.005
Vieira V, Glassmann D, Marafon P et al (2016) Effect of diclofenac sodium on seizures and inflammatory profile induced by kindling seizure model. Epilepsy Res 127:107–113. https://doi.org/10.1016/j.eplepsyres.2016.08.020
Blommel ML, Blommel AL (2007) Pregabalin: an antiepileptic agent useful for neuropathic pain. Am J Heal Pharm 64:1475–1482. https://doi.org/10.2146/ajhp060371
Nader MA, Ateyya H, El-shafey M, El-sherbeeny NA (2018) Neurochemistry international sitagliptin enhances the neuroprotective effect of pregabalin against pentylenetetrazole-induced acute epileptogenesis in mice : implication of oxidative, inflammatory, apoptotic and autophagy pathways. Neurochem Int 115:11–23. https://doi.org/10.1016/j.neuint.2017.10.006
Kruszewski SP, Shane JA (2007) Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology 68:2158–2160. https://doi.org/10.1212/01.wnl.0000269479.84817.0a
Ra M, Straube S, Pj W et al (2010) Pregabalin for acute and chronic pain in adults (review). Cochrane Libr. https://doi.org/10.1002/14651858.CD007076.pub2
Wiffen PJ, McQuay HJ, Edwards J, Moore RA (2005) Gabapentin for acute and chronic pain (review). Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD005452
Woodbury A, Yu SP, Wei L, García P (2013) Neuro-modulating effects of honokiol: a review. Front Neurol. https://doi.org/10.3389/fneur.2013.00130
Ferrendelli JA, Holland KD, McKeon AC, Covey DF (1989) Comparison of the anticonvulsant activities of ethosuximide, valproate, and a new anticonvulsant, thiobutyrolactone. Epilepsia 30:617–622. https://doi.org/10.1111/j.1528-1157.1989.tb05482.x
Holland KD, McKeon AC, Canney DJ et al (1992) Relative anticonvulsant effects of GABAmimetic and GABA modulatory agents. Epilepsia 33:981–986. https://doi.org/10.1111/j.1528-1157.1992.tb01747.x
Duke JA (1992) Handbook of biologically active phytochemicals and their activities. CRC Press, Boca Raton, pp 3–183
Viana CCS, de Oliveira PA, da Brum LF et al (2007) Gamma-decanolactone effect on behavioral and genotoxic parameters. Life Sci 80:1014–1019. https://doi.org/10.1016/j.lfs.2006.11.042
De Oliveira PA, Lino FL, Cappelari SE et al (2008) Effects of gamma-decanolactone on seizures induced by PTZ-kindling in mice. Exp Brain Res 187:161–166. https://doi.org/10.1007/s00221-008-1295-y
Pflüger P, Viau CMA, Coelho VR et al (2016) Gamma-decanolactone inhibits iNOS and TNF-alpha production by lipopolysaccharide-activated microglia in N9 cells. Eur J Pharmacol 780:38–45. https://doi.org/10.1016/j.ejphar.2016.03.029
Pflüger P, Regner GG, Coelho VR et al (2018) Gamma-decanolactone improves biochemical parameters associated with pilocarpine-induced seizures in male mice. Curr Mol Pharmacol 11:162–169. https://doi.org/10.2174/1874467210666171002114954
Pflüger P, Coelho VR, Regner GG et al (2018) Neuropharmacological profile of gamma-decanolactone on hemically-induced seizure in mice. Cent Nerv Syst Agents Med Chem 18:222–227. https://doi.org/10.2174/1871524918666180611103802
Pereira P, Elisabetsky E, Souza DO (1997) Effect of γ-decanolactone on glutamate binding in the rat cerebral cortex. Neurochem Res 22:1507–1510. https://doi.org/10.1023/A:1021962714034
Wyllie DJA, Livesey MR, Hardingham GE (2013) Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology 74:4–17. https://doi.org/10.1016/j.neuropharm.2013.01.016
Kim MJ, Dunah AW, Wang YT, Sheng M (2005) Differential roles of NR2A- and NR2B-containing NMDA receptors in ras-ERK signaling and AMPA receptor trafficking. Neuron 46:745–760. https://doi.org/10.1016/j.neuron.2005.04.031
Lai TW, Zhang S, Wang YT (2014) Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 115:157–188. https://doi.org/10.1016/j.pneurobio.2013.11.006
Waxman EA, Lynch DR (2005) N-methyl-D-aspartate receptor subtype mediated bidirectional control of p38 mitogen-activated protein kinase. J Biol Chem 280:29322–29333. https://doi.org/10.1074/jbc.M502080200
Zhou X, Ding Q, Chen Z et al (2013) Involvement of the GluN2A and GluN2B subunits in synaptic and extrasynaptic N -methyl- D -aspartate receptor function and neuronal excitotoxicity. J Biol Chem 288:24151–24159. https://doi.org/10.1074/jbc.M113.482000
Sun Y, Xu Y, Cheng X et al (2018) The differences between GluN2A and GluN2B signaling in the brain. J Neurosci Res 96:1430–1443. https://doi.org/10.1002/jnr.24251
Aronica E, Sandau US, Iyer A, Boison D (2013) Glial adenosine kinase—a neuropathological marker of the epileptic brain. Neurochem Int 63:688–695. https://doi.org/10.1016/j.neuint.2013.01.028
Boison D (2013) Role of adenosine in status epilepticus: a potential new target? Epilepsia 54:20–22. https://doi.org/10.1111/epi.12268
Ghislandi AB, Garcez ML, Zambon GM et al (2020) Adenosine and NMDA receptors modulate neuroprotection-induced NMDA preconditioning in mice. J Mol Neurosci 2:590–599
Vianna EP, Ferreira AT, Jos M (2005) Modulation of seizures and synaptic plasticity by adenosinergic receptors in an experimental model of temporal lobe epilepsy induced by pilocarpine in rats. Epilepsia 46:166–173. https://doi.org/10.1111/j.1528-1167.2005.01027.x
Zhou Q, Zhu S, Guo Y et al (2018) Adenosine A1 receptors play an important protective role against cognitive impairment and long-term potentiation inhibition in a pentylenetetrazol mouse model of epilepsy. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0571-x
Coelho De Souza GP, Elisabetsky E, Nunes DS et al (1997) Anticonvulsant properties of γ-decanolactone in mice. J Ethnopharmacol 58:175–181. https://doi.org/10.1016/S0378-8741(97)00102-5
Da Silva LF, Pereira P, Elisabetsky E (1998) A neuropharmacological analysis of PTZ-induced kindling in mice. Gen Pharmacol. https://doi.org/10.1016/S0306-3623(97)00423-0
Pflüger P, Gregory G, Luft J et al (2020) Gamma-decanolactone attenuates acute and chronic seizures in mice: a possible role of adenosine A 1 receptors. Behav Pharmacol. https://doi.org/10.1097/FBP.0000000000000554
Pérez-Saad H, Buznego MT (2008) Behavioral and antiepileptic effects of acute administration of the extract of the plant Cestrum nocturnum Lin (the lady of the night). Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2007.12.012
Gilron I, Baron R, Jensen T (2015) Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc 90:532–545. https://doi.org/10.1016/j.mayocp.2015.01.018
Enke O, New HA, New CH et al (2018) Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ 190:E786–E793. https://doi.org/10.1503/cmaj.171333
St. John Smith E (2018) Advances in understanding nociception and neuropathic pain. J Neurol 265:231–238. https://doi.org/10.1007/s00415-017-8641-6
Silva JC, Raquel S, De LG et al (2013) Modelos experimentais para avaliação da atividade antinociceptiva de produtos naturais : uma revisão. Rev Bras Farm 94:18–23
Scarabelot VL, Medeiros LF, de Oliveira C et al (2016) Melatonin alters the mechanical and thermal hyperalgesia induced by orofacial pain model in rats. Inflammation 39:1649–1659. https://doi.org/10.1007/s10753-016-0399-y
Bannon AW, Malmberg AB (2007) Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci Chapter 8:1–16. https://doi.org/10.1002/0471142301.ns0809s41
Leite JPV (2009) Fitoerapia: bases científicas e tecnológicas. Atheneu, São Paulo
Scarabelot VL, de Oliveira C, Medeiros LF et al (2019) Transcranial direct-current stimulation reduces nociceptive behavior in an orofacial pain model. J Oral Rehabil 46:40–50. https://doi.org/10.1111/joor.12726
Tice RR, Agurell E, Anderson D et al (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221. https://doi.org/10.1002/(SICI)1098-2280(2000)35:3%3c206::AID-EM8%3e3.0.CO;2-J
Antunes FTT, Angelo SG, Dallegrave E et al (2020) Recombinant peptide derived from the venom the Phoneutria nigriventer spider relieves nociception by nerve deafferentation. Neuropeptides. https://doi.org/10.1016/j.npep.2019.101980
Lazzarotto L, Pflüger P, Regner GG et al (2020) Lacosamide improves biochemical, genotoxic, and mitochondrial parameters after PTZ-kindling model in mice. Fundam Clin Pharmacol. https://doi.org/10.1111/fcp.12598
Mavournin KH, Blakey DH, Cimino MC et al (2008) The in vivo micronucleus assay in mammalian bone marrow and peripheral blood. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res Rev Genet Toxicol 239:29–80
Gregory NS, Harris AL, Robinson CR et al (2013) An overview of animal models of pain: disease models and outcome measures. J Pain 14:1255–1269. https://doi.org/10.1016/j.jpain.2013.06.008
Gunn A, Bobeck EN, Weber C, Morgan MM (2011) The influence of non-nociceptive factors on hot-plate latency in rats. J Pain. https://doi.org/10.1016/j.jpain.2010.06.011
Vendruscolo LF, Pamplona A, Takahashi RN (2004) Strain and sex differences in the expression of nociceptive behavior and stress-induced analgesia in rats. Brain Res 1030:277–283. https://doi.org/10.1016/j.brainres.2004.10.016
Kavaliers M, Douglas DC (1991) Sex differences in opioid and non-opioid mediated predator-induced analgesia in mice. Brain Res 568:173–177
Hamada NM, Ashour RH, Shalaby AA, El-Beltagi HM (2018) Calcitonin potentiates the anticonvulsant and antinociceptive effects of valproic acid and pregabalin in pentylenetetrazole-kindled mice. Eur J Pharmacol 818:351–355. https://doi.org/10.1016/j.ejphar.2017.11.003
Łuszczki JJ (2010) Dose-response relationship analysis of pregabalin doses and their antinociceptive effects in hot-plate test in mice. Pharmacol Rep 62:942–948. https://doi.org/10.1016/S1734-1140(10)70355-8
Chen T, Zhang B, Li G et al (2016) Simvastatin enhances NMDA receptor GluN2B expression and phosphorylation of GluN2B and GluN2A through increased histone acetylation and Src signaling in hippocampal CA1 neurons. Neuropharmacology 107:411–421. https://doi.org/10.1016/j.neuropharm.2016.03.028
Fujita Y, Morinobu S, Takei S et al (2012) Vorinostat, a histone deacetylase inhibitor, facilitates fear extinction and enhances expression of the hippocampal NR2B-containing NMDA receptor gene. J Psychiatr Res 46:635–643. https://doi.org/10.1016/j.jpsychires.2012.01.026
Nghia NA, Hirasawa T, Kasai H et al (2015) Long-term imipramine treatment increases N-methyl-d-aspartate receptor activity and expression via epigenetic mechanisms. Eur J Pharmacol 752:69–77. https://doi.org/10.1016/j.ejphar.2015.02.010
Morissette M, Le Saux M, D’Astous M et al (2008) Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol 108:327–338. https://doi.org/10.1016/j.jsbmb.2007.09.011
McCarthny CR, Du X, Wu YC, Hill RA (2018) Investigating the interactive effects of sex steroid hormones and brain-derived neurotrophic factor during adolescence on hippocampal NMDA receptor expression. Int J Endocrinol. https://doi.org/10.1155/2018/7231915
Adkins JM, Lynch JF, Hagerdorn P et al (2019) Anterior cingulate cortex and dorsal hippocampal glutamate receptors mediate generalized fear in female rats. Psychoneuroendocrinology 107:109–118. https://doi.org/10.1016/j.psyneuen.2019.05.009
Ospina JA, Brevig HN, Krause DN et al (2020) Estrogen suppresses IL-1 1beta -mediated induction of COX-2 pathway in rat cerebral blood vessels. Am J Physiol Heart Circ Physiol 4625:2010–2019
Stacey W, Bhave S, Uht RM (2016) Mechanisms by which 17 β -estradiol ( E2) suppress neuronal cox-2 gene expression. PLoS ONE. https://doi.org/10.1371/journal.pone.0161430
Vanore G, Giraldez L, Arnaiz GRDL, Girardi E (2001) Seizure activity produces differential changes in adenosine A 1 receptors within rat hippocampus. Neurochem Res 26:225–230
Boison D (2005) Adenosine and epilepsy: from therapeutic rationale to new therapeutic strategies. Neuroscientist 11:25–36. https://doi.org/10.1177/1073858404269112
Davoudi M, Shojaei A, Reza M et al (2013) Comparison between standard protocol and a novel window protocol for induction of pentylenetetrazol kindled seizures in the rat. Epilepsy Res 106:54–63. https://doi.org/10.1016/j.eplepsyres.2013.03.016
Kardoost M, Hajizadeh-Saffar E, Ghorbanian MT et al (2019) Genotoxicity assessment of antiepileptic drugs (AEDs) in human embryonic stem cells. Epilepsy Res. https://doi.org/10.1016/j.eplepsyres.2019.106232
Coelho VR, Prado LS, Rossato R et al (2018) A 28-day sub-acute genotoxic and behavioural assessment of garcinielliptone FC. Basic Clin Pharmacol Toxicol 123:207–212. https://doi.org/10.1111/bcpt.13010
Hayashi M (2016) The micronucleus test-most widely used in vivo genotoxicity test. Genes Environ 38:4–9. https://doi.org/10.1186/s41021-016-0044-x
Knasmüller S, Fenech M (2019) The micronucleus assay in toxicology. Royal Society of Chemistry, Cambridge
Sommer S, Buraczewska I, Kruszewski M (2020) Micronucleus assay: the state of art, and future directions. Int J Mol Sci 21:7–9. https://doi.org/10.3390/ijms21041534
Samokhina E, Samokhin A (2018) Neuropathological profile of the pentylenetetrazol (PTZ) kindling model. Int J Neurosci 128:1086–1096. https://doi.org/10.1080/00207454.2018.1481064
Funding
This research received financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 307515/2019-2), and Federal University of Rio Grande do Sul (UFRGS, 36326).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Santos, F.M., Pflüger, P.F., Lazzarotto, L. et al. Gamma-Decanolactone Alters the Expression of GluN2B, A1 Receptors, and COX-2 and Reduces DNA Damage in the PTZ-Induced Seizure Model After Subchronic Treatment in Mice. Neurochem Res 46, 2066–2078 (2021). https://doi.org/10.1007/s11064-021-03345-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03345-7