Abstract
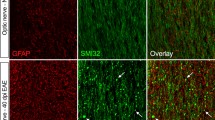
Kv1.3 is a voltage gated potassium channel that has been implicated in pathophysiology of multiple sclerosis (MS). In the present study we investigated temporal and cellular expression pattern of this channel in the lumbar part of spinal cords of animals with experimental autoimmune encephalomyelitis (EAE), animal model of MS. EAE was actively induced in female Dark Agouti rats. Expression of Kv1.3 was analyzed at different time points of disease progression, at the onset, peak and end of EAE. We here show that Kv1.3 increased by several folds at the peak of EAE at both gene and protein level. Double immunofluorescence analyses demonstrated localization of Kv1.3 on activated microglia, macrophages, and reactive astrocytes around inflammatory lesions. In vitro experiments showed that pharmacological block of Kv1.3 in activated astrocytes suppresses the expression of proinflammatory mediators, suggesting a role of this channel in inflammation. Our results support the hypothesis that Kv1.3 may be a therapeutic target of interest for MS and add astrocytes to the list of cells whose activation would be suppressed by inhibiting Kv1.3 in inflammatory conditions.








Similar content being viewed by others
References
Dendrou CA, Fugger L, Friese MA (2015) Immunopathology of multiple sclerosis. Nat Rev Immunol 15(9):545–558. https://doi.org/10.1038/nri3871
Bogie JF, Stinissen P, Hendriks JJ (2014) Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol 128(2):191–213. https://doi.org/10.1007/s00401-014-1310-2
Duffy SS, Lees JG, Moalem-Taylor G (2014) The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis. Multiple Scler Int 2014:285245. https://doi.org/10.1155/2014/285245
Barnett MH, Prineas JW (2004) Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 55(4):458–468. https://doi.org/10.1002/ana.20016
Ponomarev ED, Shriver LP, Maresz K, Dittel BN (2005) Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res 81(3):374–389. https://doi.org/10.1002/jnr.20488
Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A (2005) Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 11(2):146–152. https://doi.org/10.1038/nm1177
Nair A, Frederick TJ, Miller SD (2008) Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci 65(17):2702–2720. https://doi.org/10.1007/s00018-008-8059-5
Brosnan CF, Raine CS (2013) The astrocyte in multiple sclerosis revisited. Glia 61(4):453–465. https://doi.org/10.1002/glia.22443
Correale J, Farez MF (2015) The role of astrocytes in multiple sclerosis progression. Front Neurol 6:180. https://doi.org/10.3389/fneur.2015.00180
Ehling P, Bittner S, Budde T, Wiendl H, Meuth SG (2011) Ion channels in autoimmune neurodegeneration. FEBS Lett 585(23):3836–3842. https://doi.org/10.1016/j.febslet.2011.03.065
Rangaraju S, Chi V, Pennington MW, Chandy KG (2009) Kv1.3 potassium channels as a therapeutic target in multiple sclerosis. Expert Opin Ther Targets 13(8):909–924. https://doi.org/10.1517/14728220903018957
Wulff H, Castle NA, Pardo LA (2009) Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov 8(12):982–1001. https://doi.org/10.1038/nrd2983
Wulff H, Calabresi PA, Allie R, Yun S, Pennington M, Beeton C, Chandy KG (2003) The voltage-gated Kv1.3 K+ channel in effector memory T cells as new target for MS. J Clin Invest 111(11):1703–1713. https://doi.org/10.1172/jci16921
Panyi G, Vamosi G, Bacso Z, Bagdany M, Bodnar A, Varga Z, Gaspar R, Matyus L, Damjanovich S (2004) Kv1.3 potassium channels are localized in the immunological synapse formed between cytotoxic and target cells. Proc Natl Acad Sci USA 101(5):1285–1290. https://doi.org/10.1073/pnas.0307421100
Rus H, Pardo CA, Hu L, Darrah E, Cudrici C, Niculescu T, Niculescu F, Mullen KM, Allie R, Guo L, Wulff H, Beeton C, Judge SI, Kerr DA, Knaus HG, Chandy KG, Calabresi PA (2005) The voltage-gated potassium channel Kv1.3 is highly expressed on inflammatory infiltrates in multiple sclerosis brain. Proc Natl Acad Sci USA 102(31):11094–11099. https://doi.org/10.1073/pnas.0501770102
Wulff H, Knaus HG, Pennington M, Chandy KG (2004) K+ channel expression during B Cell differentiation: implications for immunomodulation and autoimmunity. J Immunol 173(2):776–786. https://doi.org/10.4049/jimmunol.173.2.776
de la Cruz A, Vera-Zambrano A, Peraza DA, Valenzuela C, Zapata JM, Perez-Chacon G, Gonzalez T (2017) Fludarabine inhibits KV1.3 currents in human B lymphocytes. Front Pharmacol 8:177. https://doi.org/10.3389/fphar.2017.00177
Vicente R, Escalada A, Coma M, Fuster G, Sanchez-Tillo E, Lopez-Iglesias C, Soler C, Solsona C, Celada A, Felipe A (2003) Differential voltage-dependent K+ channel responses during proliferation and activation in macrophages. J Biol Chem 278(47):46307–46320. https://doi.org/10.1074/jbc.M304388200
Villalonga N, Escalada A, Vicente R, Sanchez-Tillo E, Celada A, Solsona C, Felipe A (2007) Kv1.3/Kv1.5 heteromeric channels compromise pharmacological responses in macrophages. Biochem Biophys Res Commun 352(4):913–918. https://doi.org/10.1016/j.bbrc.2006.11.120
Mullen KM, Rozycka M, Rus H, Hu L, Cudrici C, Zafranskaia E, Pennington MW, Johns DC, Judge SI, Calabresi PA (2006) Potassium channels Kv1.3 and Kv1.5 are expressed on blood-derived dendritic cells in the central nervous system. Ann Neurol 60(1):118–127. https://doi.org/10.1002/ana.20884
Matzner N, Zemtsova IM, Xuan NT, Duszenko M, Shumilina E, Lang F (2008) Ion channels modulating mouse dendritic cell functions. J Immunol 181(10):6803–6809. https://doi.org/10.4049/jimmunol.181.10.6803
Khanna R, Roy L, Zhu X, Schlichter LC (2001) K+ channels and the microglial respiratory burst. Am J Physiol Cell Physiol 280(4):C796–C806
Fordyce CB, Jagasia R, Zhu X, Schlichter LC (2005) Microglia Kv1.3 channels contribute to their ability to kill neurons. J Neurosci 25(31):7139–7149. https://doi.org/10.1523/JNEUROSCI.1251-05.2005
Rangaraju S, Gearing M, Jin LW, Levey A (2015) Potassium channel Kv1.3 is highly expressed by microglia in human Alzheimer’s disease. J Alzheimer’s Dis 44(3):797–808. https://doi.org/10.3233/JAD-141704
Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O (1995) Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur J Neurosci 7(11):2189–2205
Ramirez-Navarro A, Glazebrook PA, Kane-Sutton M, Padro C, Kline DD, Kunze DL (2011) Kv1.3 channels regulate synaptic transmission in the nucleus of solitary tract. J Neurophysiol 105(6):2772–2780. https://doi.org/10.1152/jn.00494.2010
Vicente R, Escalada A, Villalonga N, Texido L, Roura-Ferrer M, Martin-Satue M, Lopez-Iglesias C, Soler C, Solsona C, Tamkun MM, Felipe A (2006) Association of Kv1.5 and Kv1.3 contributes to the major voltage-dependent K+ channel in macrophages. J Biol Chem 281(49):37675–37685. https://doi.org/10.1074/jbc.M605617200
Chung I, Zelivyanskaya M, Gendelman HE (2002) Mononuclear phagocyte biophysiology influences brain transendothelial and tissue migration: implication for HIV-1-associated dementia. J Neuroimmunol 122(1–2):40–54
Stebbing MJ, Cottee JM, Rana I (2015) The role of ion channels in microglial activation and proliferation—a complex interplay between ligand-gated ion channels, K(+) channels, and intracellular Ca(2+.). Front Immunol 6:497. https://doi.org/10.3389/fimmu.2015.00497
Kotecha SA, Schlichter LC (1999) A Kv1.5 to Kv1.3 switch in endogenous hippocampal microglia and a role in proliferation. J Neurosci 19(24):10680–10693
Nutile-McMenemy N, Elfenbein A, Deleo JA (2007) Minocycline decreases in vitro microglial motility, beta1-integrin, and Kv1.3 channel expression. J Neurochem 103(5):2035–2046. https://doi.org/10.1111/j.1471-4159.2007.04889.x
Charolidi N, Schilling T, Eder C (2015) Microglial Kv1.3 channels and P2Y12 receptors differentially regulate cytokine and chemokine release from brain slices of young adult and aged mice. PLoS ONE 10(5):e0128463. https://doi.org/10.1371/journal.pone.0128463
Huang J, Han S, Sun Q, Zhao Y, Liu J, Yuan X, Mao W, Peng B, Liu W, Yin J, He X (2017) Kv1.3 channel blocker (ImKTx88) maintains blood-brain barrier in experimental autoimmune encephalomyelitis. Cell Biosci 7:31. https://doi.org/10.1186/s13578-017-0158-2
Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts DR, Wang PH, LeeHealey CJ, Andrews BS, Sankaranarayanan A, Homerick D, Roeck WW, Tehranzadeh J, Stanhope KL, Zimin P, Havel PJ, Griffey S, Knaus HG, Nepom GT, Gutman GA, Calabresi PA, Chandy KG (2006) Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci USA 103(46):17414–17419. https://doi.org/10.1073/pnas.0605136103
Lam J, Wulff H (2011) The lymphocyte potassium channels Kv1.3 and KCa3.1 as targets for immunosuppression. Drug Dev Res 72(7):573–584. https://doi.org/10.1002/ddr.20467
Richner M, Jager SB, Siupka P, Vaegter CB (2017) Hydraulic extrusion of the spinal cord and isolation of dorsal root ganglia in rodents. J Vis Exp. https://doi.org/10.3791/55226
Lavrnja I, Laketa D, Savic D, Bozic I, Bjelobaba I, Pekovic S, Nedeljkovic N (2015) Expression of a second ecto-5′-nucleotidase variant besides the usual protein in symptomatic phase of experimental autoimmune encephalomyelitis. J Mol Neurosci 55(4):898–911. https://doi.org/10.1007/s12031-014-0445-x
Hewitt SM, Baskin DG, Frevert CW, Stahl WL, Rosa-Molinar E (2014) Controls for immunohistochemistry: the histochemical society’s standards of practice for validation of immunohistochemical assays. J Histochem Cytochem 62(10):693–697. https://doi.org/10.1369/0022155414545224
Burry RW (2011) Controls for immunocytochemistry: an update. J Histochem Cytochem 59(1):6–12. https://doi.org/10.1369/jhc.2010.956920
Jakovljevic M, Lavrnja I, Bozic I, Savic D, Bjelobaba I, Pekovic S, Sevigny J, Nedeljkovic N, Laketa D (2017) Down-regulation of NTPDase2 and ADP-sensitive P2 purinoceptors correlate with severity of symptoms during experimental autoimmune encephalomyelitis. Front Cell Neurosci 11:333. https://doi.org/10.3389/fncel.2017.00333
Parker KK, Norenberg MD, Vernadakis A (1980) “Transdifferentiation” of C6 glial cells in culture. Science 208(4440):179–181
Quincozes-Santos A, Bobermin LD, Latini A, Wajner M, Souza DO, Goncalves CA, Gottfried C (2013) Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS ONE 8(5):e64372. https://doi.org/10.1371/journal.pone.0064372
Nardin P, Tramontina F, Leite MC, Tramontina AC, Quincozes-Santos A, de Almeida LM, Battastini AM, Gottfried C, Goncalves CA (2007) S100B content and secretion decrease in astrocytes cultured in high-glucose medium. Neurochem Int 50(5):774–782. https://doi.org/10.1016/j.neuint.2007.01.013
Loureiro SO, Heimfarth L, de Lima BO, Leite MC, Guerra MC, Goncalves CA, Pessoa-Pureur R (2012) Dual action of chronic ethanol treatment on LPS-induced response in C6 glioma cells. J Neuroimmunol 249(1–2):8–15. https://doi.org/10.1016/j.jneuroim.2012.04.004
Suk K, Lee J, Hur J, Kim YS, Lee M, Cha S, Yeou Kim S, Kim H (2001) Activation-induced cell death of rat astrocytes. Brain Res 900(2):342–347
Do H, Pyo S, Sohn EH (2010) Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-alpha- and IFN-gamma-stimulated C6 glioma cells. J Nutr Biochem 21(8):671–679. https://doi.org/10.1016/j.jnutbio.2009.03.013
Beeton C, Barbaria J, Giraud P, Devaux J, Benoliel AM, Gola M, Sabatier JM, Bernard D, Crest M, Beraud E (2001) Selective blocking of voltage-gated K+ channels improves experimental autoimmune encephalomyelitis and inhibits T cell activation. J Immunol 166(2):936–944. https://doi.org/10.4049/jimmunol.166.2.936
Beeton C, Wulff H, Barbaria J, Clot-Faybesse O, Pennington M, Bernard D, Cahalan MD, Chandy KG, Beraud E (2001) Selective blockade of T lymphocyte K(+) channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc Natl Acad Sci USA 98(24):13942–13947. https://doi.org/10.1073/pnas.241497298
Beeton C, Pennington MW, Wulff H, Singh S, Nugent D, Crossley G, Khaytin I, Calabresi PA, Chen CY, Gutman GA, Chandy KG (2005) Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol Pharmacol 67(4):1369–1381. https://doi.org/10.1124/mol.104.008193
Judge SI, Yeh JZ, Mannie MD, Pope Seifert L, Paterson PY (1997) Potassium channel blockers inhibit adoptive transfer of experimental allergic encephalomyelitis by myelin-basic-protein-stimulated rat T lymphocytes. J Biomed Sci 4(4):169–178
Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, de la Maza LM, Monuki E, Flugel A, Pennington MW, Parker I, Chandy KG, Cahalan MD (2008) Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity 29(4):602–614. https://doi.org/10.1016/j.immuni.2008.07.015
Gocke AR, Lebson LA, Grishkan IV, Hu L, Nguyen HM, Whartenby KA, Chandy KG, Calabresi PA (2012) Kv1.3 deletion biases T cells toward an immunoregulatory phenotype and renders mice resistant to autoimmune encephalomyelitis. J Immunol 188(12):5877–5886. https://doi.org/10.4049/jimmunol.1103095
Utsunomiya I, Yoshihashi E, Tanabe S, Nakatani Y, Ikejima H, Miyatake T, Hoshi K, Taguchi K (2008) Expression and localization of Kv1 potassium channels in rat dorsal and ventral spinal roots. Exp Neurol 210(1):51–58. https://doi.org/10.1016/j.expneurol.2007.09.032
Vicente R, Villalonga N, Calvo M, Escalada A, Solsona C, Soler C, Tamkun MM, Felipe A (2008) Kv1.5 association modifies Kv1.3 traffic and membrane localization. J Biol Chem 283(13):8756–8764. https://doi.org/10.1074/jbc.M708223200
Yang Y, Wang YF, Yang XF, Wang ZH, Lian YT, Yang Y, Li XW, Gao X, Chen J, Shu YW, Cheng LX, Liao YH, Liu K (2013) Specific Kv1.3 blockade modulates key cholesterol-metabolism-associated molecules in human macrophages exposed to ox-LDL. J Lipid Res 54(1):34–43. https://doi.org/10.1194/jlr.M023846
Lavrnja I, Smiljanic K, Savic D, Mladenovic-Djordjevic A, Tesovic K, Kanazir S, Pekovic S (2017) Expression profiles of cholesterol metabolism-related genes are altered during development of experimental autoimmune encephalomyelitis in the rat spinal cord. Sci Rep 7(1):2702. https://doi.org/10.1038/s41598-017-02638-8
Boven LA, Van Meurs M, Van Zwam M, Wierenga-Wolf A, Hintzen RQ, Boot RG, Aerts JM, Amor S, Nieuwenhuis EE, Laman JD (2006) Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 129(Pt 2):517–526. https://doi.org/10.1093/brain/awh707
Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P, Amor S, Teunissen CE, van Horssen J, Dijkstra CD (2013) Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflamm 10:35. https://doi.org/10.1186/1742-2094-10-35
Rangaraju S, Raza SA, Pennati A, Deng Q, Dammer EB, Duong D, Pennington MW, Tansey MG, Lah JJ, Betarbet R, Seyfried NT, Levey AI (2017) A systems pharmacology-based approach to identify novel Kv1.3 channel-dependent mechanisms in microglial activation. J Neuroinflamm 14(1):128. https://doi.org/10.1186/s12974-017-0906-6
Nguyen HM, Grossinger EM, Horiuchi M, Davis KW, Jin LW, Maezawa I, Wulff H (2017) Differential Kv1.3, KCa3.1, and Kir2.1 expression in “classically” and “alternatively” activated microglia. Glia 65(1):106–121. https://doi.org/10.1002/glia.23078
Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, Bethea JR (2014) Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia 62(3):452–467. https://doi.org/10.1002/glia.22616
Bjelobaba I, Savic D, Lavrnja I (2017) Multiple sclerosis and neuroinflammation: the overview of current and prospective therapies. Curr Pharm Des 23(5):693–730. https://doi.org/10.2174/1381612822666161214153108
Pekny M (2001) Astrocytic intermediate filaments: lessons from GFAP and vimentin knock-out mice. Prog Brain Res 132:23–30. https://doi.org/10.1016/s0079-6123(01)32062-9
Pekny M, Pekna M (2004) Astrocyte intermediate filaments in CNS pathologies and regeneration. J Pathol 204(4):428–437. https://doi.org/10.1002/path.1645
Aquino DA, Chiu FC, Brosnan CF, Norton WT (1988) Glial fibrillary acidic protein increases in the spinal cord of Lewis rats with acute experimental autoimmune encephalomyelitis. J Neurochem 51(4):1085–1096
Zhu J, Yan J, Thornhill WB (2014) The Kv1.3 potassium channel is localized to the cis-Golgi and Kv1.6 is localized to the endoplasmic reticulum in rat astrocytes. FEBS J 281(15):3433–3445. https://doi.org/10.1111/febs.12871
Reeves TM, Trimmer PA, Colley BS, Phillips LL (2016) Targeting Kv1.3 channels to reduce white matter pathology after traumatic brain injury. Exp Neurol 283(Pt A):188–203. https://doi.org/10.1016/j.expneurol.2016.06.011
Tsukada N, Miyagi K, Matsuda M, Yanagisawa N, Yone K (1991) Tumor necrosis factor and interleukin-1 in the CSF and sera of patients with multiple sclerosis. J Neurol Sci 104(2):230–234
Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W (2000) Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 123(Pt 6):1174–1183
Korn T, Magnus T, Jung S (2005) Autoantigen specific T cells inhibit glutamate uptake in astrocytes by decreasing expression of astrocytic glutamate transporter GLAST: a mechanism mediated by tumor necrosis factor-alpha. FASEB J 19(13):1878–1880. https://doi.org/10.1096/fj.05-3748fje
Mayo L, Quintana FJ, Weiner HL (2012) The innate immune system in demyelinating disease. Immunol Rev 248(1):170–187. https://doi.org/10.1111/j.1600-065X.2012.01135.x
Carrillo-de Sauvage MA, Gomez A, Ros CM, Ros-Bernal F, Martin ED, Perez-Valles A, Gallego-Sanchez JM, Fernandez-Villalba E, Barcia C Sr., Barcia C Jr., Herrero MT (2012) CCL2-expressing astrocytes mediate the extravasation of T lymphocytes in the brain. Evidence from patients with glioma and experimental models in vivo. PLoS ONE 7(2):e30762. https://doi.org/10.1371/journal.pone.0030762
Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM (2001) Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med 193(6):713–726
Acknowledgements
Some data sets included in this manuscript were presented as an abstract at the 4th Meeting of COST Action BM1406: Ion Channels and Immune Response toward a global understanding of immune cell physiology and for new therapeutic approaches (IONCHAN-IMMUNRESPON).
Funding
This work was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Grant III 41014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest regarding the publication of this paper.
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of Ethical Committee for the Use of Laboratory Animals of Institute for Biological Research “Sinisa Stankovic” (Belgrade, Serbia), in compliance with EEC Directive (2010/63/EU) on the protection of animals used for experimental and other scientific purposes.
Rights and permissions
About this article
Cite this article
Bozic, I., Tesovic, K., Laketa, D. et al. Voltage Gated Potassium Channel Kv1.3 Is Upregulated on Activated Astrocytes in Experimental Autoimmune Encephalomyelitis. Neurochem Res 43, 1020–1034 (2018). https://doi.org/10.1007/s11064-018-2509-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2509-8