Abstract
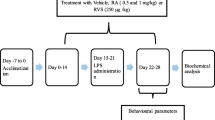
Bacterial endotoxin lipopolysaccharide (LPS) can induce systemic inflammation, and therefore disrupt learning and memory processes. Ginsenoside Rg1, a major bioactive component of ginseng, is shown to greatly improve cognitive function. The present study was designed to further investigate whether administration of ginsenoside Rg1 can ameliorate LPS-induced cognitive impairment in the Y-maze and Morris water maze (MWM) task, and to explore the underlying mechanisms. Results showed that exposure to LPS (500 μg/kg) significantly impaired working and spatial memory and that repeated treatment with ginsenoside Rg1 (200 mg/kg/day, for 30 days) could effectively alleviate the LPS-induced cognitive decline as indicated by increased working and spatial memory in the Y-maze and MWM tests. Furthermore, ginsenoside Rg1 treatment prevented LPS-induced decrease of acetylcholine (ACh) levels and increase of acetylcholinesterase (AChE) activity. Ginsenoside Rg1 treatment also reverted the decrease of alpha7 nicotinic acetylcholine receptor (α7 nAChR) protein expression in the prefrontal cortex (PFC) and hippocampus of LPS-treated rats. These findings suggest that ginsenoside Rg1 has protective effect against LPS-induced cognitive deficit and that prevention of LPS-induced changes in cholinergic system is crucial to this ameliorating effect.





Similar content being viewed by others
References
Anaeigoudari A, Soukhtanloo M, Shafei MN, Sadeghnia HR, Reisi P, Beheshti F et al (2016) Neuronal nitric oxide synthase has a role in the detrimental effects of lipopolysaccharide on spatial memory and synaptic plasticity in rats. Pharmacol Rep 68:243–249
Dehkordi NG, Noorbakhshnia M, Ghaedi K, Esmaeili A, Dabaghi M (2015) Omega-3 fatty acids prevent LPS-induced passive avoidance learning and memory and CaMKII-α gene expression impairments in hippocampus of rat. Pharmacol Rep 67:370–375
Belarbi K, Jopson T, Tweedie D, Arellano C, Luo W, Greig NH et al (2012) TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflamm 9:23
Zhou T, Zu G, Zhang X, Wang X, Li S, Gong X et al (2016) Neuroprotective effects of ginsenoside Rg1 through the Wnt/β-catenin signaling pathway in both in vivo and in vitro models of Parkinson’s disease. Neuropharmacology 101:480–489
Zhang Y, Zhang Z, Wang H, Cai N, Zhou S, Zhao Y et al (2016) Neuroprotective effect of ginsenoside Rg1 prevents cognitive impairment induced by isoflurane anesthesia in aged rats via antioxidant, anti-inflammatory and anti-apoptotic effects mediated by the PI3K/AKT/GSK-3β pathway. Mol Med Rep 14:2778–2784
Leung KW, Cheng YK, Mak NK, Chan KK, Fan TP, Wong RN (2006) Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett 580:3211–3216
Wu CF, Bi XL, Yang JY, Zhan JY, Dong YX, Wang JH et al (2007) Differential effects of ginsenosides on NO and TNF-α production by LPS-activated N9 microglia. Int Immunopharmacol 7:313–320
Bao S, Zou Y, Wang B, Li Y, Zhu J, Luo Y et al (2015) Ginsenoside Rg1 improves lipopolysaccharide-induced acute lung injury by inhibiting inflammatory responses and modulating infiltration of M2 macrophages. Int Immunopharmacol 28:429–434
Song X-Y, Hu J-F, Chu S-F, Zhang Z, Xu S, Yuan Y-H et al (2013) Ginsenoside Rg1 attenuates okadaic acid induced spatial memory impairment by the GSK3β/tau signaling pathway and the Aβ formation prevention in rats. Eur J Pharmacol 710:29–38
Shi YQ, Huang TW, Chen LM, Pan XD, Zhang J, Zhu YG et al (2010) Ginsenoside Rg1 attenuates amyloid-beta content, regulates PKA/CREB activity, and improves cognitive performance in SAMP8 mice. J Alzheimers Dis 19:977–989
Zhu G, Wang Y, Li J, Wang J (2015) Chronic treatment with ginsenoside Rg1 promotes memory and hippocampal long-term potentiation in middle-aged mice. Neuroscience 292:81–89
Robinson L, Platt B, Riedel G (2011) Involvement of the cholinergic system in conditioning and perceptual memory. Behav Brain Res 221:443–465
Takase K, Mitsushima D, Masuda J, Mogi K, Funabashi T, Endo Y et al (2005) Feeding with powdered diet after weaning affects sex difference in acetylcholine release in the hippocampus in rats. Neuroscience 136:593–599
Kart E, Jocham G, Müller CP, Schlömer C, Brandão ML, Huston JP et al (2004) Neurokinin-1 receptor antagonism by SR140333: enhanced in vivo ACh in the hippocampus and promnestic post-trial effects. Peptides 25:1959–1969
Xu Q-Q, Xu Y-J, Yang C, Tang Y, Li L, Cai H-B et al (2016) Sodium tanshinone IIA sulfonate attenuates scopolamine-induced cognitive dysfunctions via improving cholinergic system. BioMed Res Int 2016:9852536
Shen J-x, Tu B, Yakel JL (2009) Inhibition of α7-containing nicotinic ACh receptors by muscarinic M(1) ACh receptors in rat hippocampal CA1 interneurones in slices. J Physiol 587:1033–1042
Li D-J, Tang Q, Shen F-M, Su D-F, Duan J-L, Xi T (2009) Overexpressed α7 nicotinic acetylcholine receptor inhibited proinflammatory cytokine release in NIH3T3 cells. J Biosci Bioeng 108:85–91
Lykhmus O, Voytenko L, Koval L, Mykhalskiy S, Kholin V, Peschana K et al (2015) α7 Nicotinic acetylcholine receptor-specific antibody induces inflammation and amyloid β(42) accumulation in the mouse brain to impair memory. PLoS ONE 10:e0122706
Marubio LM, Paylor R (2004) Impaired passive avoidance learning in mice lacking central neuronal nicotinic acetylcholine receptors. Neuroscience 129:575–582
de Oliveira P, Gomes AQ, Pacheco TR, Vitorino de Almeida V, Saldanha C, Calado A (2012) Cell-specific regulation of acetylcholinesterase expression under inflammatory conditions. Clin Hemorheol Microcirc 51:129–137
Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J et al (2004) Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem 89:337–343
Lykhmus O, Gergalova G, Zouridakis M, Tzartos S, Komisarenko S, Skok M (2015) Inflammation decreases the level of alpha7 nicotinic acetylcholine receptors in the brain mitochondria and makes them more susceptible to apoptosis induction. Int Immunopharmacol 29:148–151
Lee Y-J, Choi D-Y, Choi IS, Kim KH, Kim YH, Kim HM et al (2012) Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models. J Neuroinflammation 9:35
Watson C, Paxinos G (2014) The rat brain in stereotaxic coordinates. Seventh Edition, Elsevier Academic Press, San Diego
Sarter M, Bodewitz G, Stephens DN (1988) Attenuation of scopolamine-induced impairment of spontaneous alteration behaviour by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology 94:491–495
Lee Y, Oliynyk S, Jung J-C, Han JJ, Oh S (2013) Administration of glucosylceramide ameliorated the memory impairment in aged mice. Evid-based Complementary Alter Med 824120:10
Hasan W, Woodward WR, Habecker BA (2012) Altered atrial neurotransmitter release in transgenic p75 –/– and gp130 KO mice. Neurosci Lett 529:55–59
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Wang X, Yan S, Wang A, Li Y, Zhang F (2014) Gastrodin ameliorates memory deficits in 3,3′-iminodipropionitrile-induced rats: possible involvement of dopaminergic system. Neurochem Res 39:1458–1466
Hritcu L, Foyet HS, Stefan M, Mihasan M, Asongalem AE, Kamtchouing P (2011) Neuroprotective effect of the methanolic extract of Hibiscus asper leaves in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. J Ethnopharmacol 137:585–591
Sasaki-Hamada S, Hoshi M, Niwa Y, Ueda Y, Kokaji A, Kamisuki S et al (2016) Neoechinulin A induced memory improvements and antidepressant-like effects in mice. Prog Neuropsychopharmacol Biol Psychiatry 71:155–161
Joshi R, Garabadu D, Teja GR, Krishnamurthy S (2014) Silibinin ameliorates LPS-induced memory deficits in experimental animals. Neurobiol Learn Mem 116:117–131
Lynch G, Palmer LC, Gall CM (2011) The likelihood of cognitive enhancement. Pharmacol Biochem Behav 99:116–129
Nizri E, Hamra-Amitay Y, Sicsic C, Lavon I, Brenner T (2006) Anti-inflammatory properties of cholinergic up-regulation: a new role for acetylcholinesterase inhibitors. Neuropharmacology 50:540–547
Silva-Herdade AS, Saldanha C (2013) Effects of acetylcholine on an animal mode of inflammation. Clin Hemorheol Microcirc 53:209–216
Tyagi E, Agrawal R, Nath C, Shukla R (2008) Influence of LPS-induced neuroinflammation on acetylcholinesterase activity in rat brain. J Neuroimmunol 205:51–56
Taepavarapruk P, Song C (2010) Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1beta administrations: effects of omega-3 fatty acid EPA treatment. J Neurochem 112:1054–1064
Zong Y, Ai QL, Zhong LM, Dai JN, Yang P, He Y et al (2012) Ginsenoside Rg1 attenuates lipopolysaccharide-induced inflammatory responses via the phospholipase Cγ signaling pathway in murine BV-2 microglial cells. Curr Med Chem 19:770–779
Wang Q, Sun LH, Jia W, Liu XM, Dang HX, Mai WL et al (2010) Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamine-induced learning and memory impairment in mice. Phytother Res 24:1748–1754
Kim JM, Park SK, Guo TJ, Kang JY, Ha JS, Lee DS et al (2016) Anti-amnesic effect of Dendropanax morbifera via JNK signaling pathway on cognitive dysfunction in high-fat diet-induced diabetic mice. Behav Brain Res 312:39–54
Pehrson AL, Hillhouse TM, Haddjeri N, Rovera R, Porter JH, Mørk A et al (2016) Task- and treatment length-dependent effects of vortioxetine on scopolamine-induced cognitive dysfunction and hippocampal extracellular acetylcholine in rats. J Pharmacol Exp Ther 358:472–482
Deibel SH, Weishaupt N, Regis AM, Hong NS, Keeley RJ, Balog RJ et al (2016) Subtle learning and memory impairment in an idiopathic rat model of Alzheimer’s disease utilizing cholinergic depletions and β-amyloid. Brain Res 1646:12–24
Yakel JL (2014) Nicotinic ACh receptors in the hippocampal circuit; functional expression and role in synaptic plasticity. J Physiol 592:4147–4153
Cao G, Zhu J, Zhong Q, Shi C, Dang Y, Han W et al (2013) Distinct roles of methamphetamine in modulating spatial memory consolidation, retrieval, reconsolidation and the accompanying changes of ERK and CREB activation in hippocampus and prefrontal cortex. Neuropharmacology 67:144–154
Churchwell JC, Kesner RP (2011) Hippocampal-prefrontal dynamics in spatial working memory: Interactions and independent parallel processing. Behav Brain Res 225:389–395
Li Y, Liu L, Kang J, Sheng JG, Barger SW, Mrak RE et al (2000) Neuronal-glial interactions mediated by interleukin-1 enhance neuronal acetylcholinesterase activity and mRNA expression. J Neurosci 20:149–155
Ming Z, Wotton CA, Appleton RT, Ching JC, Loewen ME, Sawicki G et al (2015) Systemic lipopolysaccharide-mediated alteration of cortical neuromodulation involves increases in monoamine oxidase-A and acetylcholinesterase activity. J Neuroinflamm 12:37
Chiapinotto Spiazzi C, Bucco Soares M, Pinto Izaguirry A, Musacchio Vargas L, Zanchi MM, Frasson Pavin N et al (2015) Selenofuranoside ameliorates memory loss in Alzheimer-like sporadic dementia: AChE activity, oxidative stress, and inflammation involvement. Oxid Med Cell Longev 2015:976908
Kim E-J, Jung I-H, Van Le TK, Jeong J-J, Kim N-J, Kim D-H (2013) Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. J Ethnopharmacol 146:294–299
Ming Z, Sawicki G, Bekar LK (2015) Acute systemic LPS-mediated inflammation induces lasting changes in mouse cortical neuromodulation and behavior. Neurosci Lett 590:96–100
Chu S, Gu J, Feng L, Liu J, Zhang M, Jia X et al (2014) Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int Immunopharmacol 19:317–326
Foyet HS, Ngatanko Abaïssou HH, Wado E, Asongalem Acha E, Alin C (2015) Emilia coccinae (SIMS) G Extract improves memory impairment, cholinergic dysfunction, and oxidative stress damage in scopolamine-treated rats. BMC Complement Altern Med 15:333
Tyagi E, Agrawal R, Nath C, Shukla R (2010) Inhibitory role of cholinergic system mediated via α7 nicotinic acetylcholine receptor in LPS-induced neuro-inflammation. Innate. Immunity 16:3–13
Lykhmus O, Mishra N, Koval L, Kalashnyk O, Gergalova G, Uspenska K et al (2016) Molecular mechanisms regulating LPS-Induced inflammation in the brain. Front Mol Neurosci 9:19
Levin ED, McClernon FJ, Rezvani AH (2006) Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology 184:523–539
Chen T, Wang C, Sha S, Zhou L, Chen L, Chen L (2016) Simvastatin enhances spatial memory and long-term potentiation in hippocampal CA1 via upregulation of α7 nicotinic acetylcholine receptor. Mol Neurobiol 53:4060–4072
Acknowledgements
This work was supported by the Provincial Natural Science Foundation of Shandong [Grant Number ZR2015HQ002], the National Natural Science Foundation of China [Grant Numbers 81572534 and 81602226], the Provincial Projects of Medical and Technology Development Program of Shandong [Grant Numbers 2014WS0091 and 2014WS0347], the Key Research and Development Program of Shandong Province [Grant Number 2015GSF118096], and a China Postdoctoral Science Foundation Funded Project [Grant Number 2016M590641].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jin, Y., Peng, J., Wang, X. et al. Ameliorative Effect of Ginsenoside Rg1 on Lipopolysaccharide-Induced Cognitive Impairment: Role of Cholinergic System. Neurochem Res 42, 1299–1307 (2017). https://doi.org/10.1007/s11064-016-2171-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2171-y