Abstract
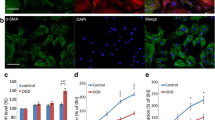
Cerebral ischemia/reperfusion is known to activate endogenous neural stem/progenitor cell (NS/PC) proliferation, but the mechanisms leading to NS/PC proliferation remain unknown. Astrocytes are vital components of the neurogenic niche and play a crucial role in regulating NS/PC proliferation and differentiation. After focal cerebral ischemia/reperfusion (I/R), astrocytes release a damage-associated molecular-pattern molecule called high-mobility group box 1 (HMGB1). Since HMGB1 is critical for NS/PC proliferation during brain development, we modeled I/R using glucose deprivation/reperfusion (OGD/R) in vitro and examined the effect of HMGB1 released by astrocytes on NS/PC proliferation. Further, we determined the role of the PI3K/Akt signaling pathway in this process. Using conditioned media from OGD/R astrocytes with or without RNA interference for HMGB1, as well as with anti-HMGB1 antibodies, we evaluated the effect of astrocyte-derived HMGB1 on NS/PC proliferation. Using the potent PI3K/Akt inhibitor, LY294002, we explored the likely mechanism of HMGB1-induced NS/PC proliferation. OGD/R astrocyte-conditioned media (ACM) increased NS/PC proliferation, and HMGB1 RNA interference prevented this effect. Using an HMGB1 neutralizing antibody in OGD/R ACM also abrogated NS/PC proliferation. LY294002 effectively reduced phospho-Akt levels and reduced NS/PC proliferation induced by HMGB1 in vitro. Our data demonstrate that HMGB1 released by OGD/R astrocytes promotes NS/PC proliferation through activation of the PI3K/Akt signaling pathway. Local HMGB1 release may induce endogenous NS/PC to proliferate following cerebral I/R and suggests that HMGB1 may play a pivotal role in brain tissue repair after an ischemic event.





Similar content being viewed by others
References
Nakagomi T, Taguchi A, Fujimori Y, Saino O, Nakano-Doi A, Kubo S, Gotoh A, Soma T, Yoshikawa H, Nishizaki T, Nakagomi N, Stern DM, Matsuyama T (2009) Isolation and characterization of neural stem/progenitor cells from post-stroke cerebral cortex in mice. Eur J Neurosci 29:1842–1852
Sims JR, Lee SW, Topalkara K, Qiu J, Xu J, Zhou Z, Moskowitz MA (2009) Sonic hedgehog regulates ischemia/hypoxia-induced neural progenitor proliferation. Stroke 40:3618–3626
Cao X, Li LP, Qin XH, Li SJ, Zhang M, Wang Q, Hu HH, Fang YY, Gao YB, Li XW, Sun LR, Xiong WC, Gao TM, Zhu XH (2013) Astrocytic adenosine 5′-triphosphate release regulates the proliferation of neural stem cells in the adult hippocampus. Stem Cells 31:1633–1643
Go HS, Shin CY, Lee SH, Jeon SJ, Kim KC, Choi CS, Ko KH (2009) Increased proliferation and gliogenesis of cultured rat neural progenitor cells by lipopolysaccharide-stimulated astrocytes. NeuroImmunoModulation 16:365–376
Lee C, Hu J, Ralls S, Kitamura T, Loh YP, Yang Y, Mukouyama YS, Ahn S (2012) The molecular profiles of neural stem cell niche in the adult subventricular zone. PLoS One 7:e50501
Sirko S, Behrendt G, Johansson PA, Tripathi P, Costa M, Bek S, Heinrich C, Tiedt S, Colak D, Dichgans M, Fischer IR, Plesnila N, Staufenbiel M, Haass C, Snapyan M, Saghatelyan A, Tsai LH, Fischer A, Grobe K, Dimou L, Gotz M (2013) Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. [corrected]. Cell Stem Cell 12:426–439
Hayakawa K, Pham LD, Katusic ZS, Arai K, Lo EH (2012) Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci USA 109:7505–7510
Kim SW, Jin Y, Shin JH, Kim ID, Lee HK, Park S, Han PL, Lee JK (2012) Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion. Neurobiol Dis 46:147–156
Zhang J, Takahashi HK, Liu K, Wake H, Liu R, Maruo T, Date I, Yoshino T, Ohtsuka A, Mori S, Nishibori M (2011) Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke 42:1420–1428
Kim SW, Lim CM, Kim JB, Shin JH, Lee S, Lee M, Lee JK (2011) Extracellular HMGB1 released by NMDA treatment confers neuronal apoptosis via RAGE-p38 MAPK/ERK signaling pathway. Neurotox Res 20:159–169
Guazzi S, Strangio A, Franzi AT, Bianchi ME (2003) HMGB1, an architectural chromatin protein and extracellular signalling factor, has a spatially and temporally restricted expression pattern in mouse brain. Gene Expr Patterns 3:29–33
Meneghini V, Bortolotto V, Francese MT, Dellarole A, Carraro L, Terzieva S, Grilli M (2013) High-mobility group box-1 protein and beta-amyloid oligomers promote neuronal differentiation of adult hippocampal neural progenitors via receptor for advanced glycation end products/nuclear factor-kappaB axis: relevance for Alzheimer’s disease. J Neurosci 33:6047–6059
Zhao X, Kuja-Panula J, Rouhiainen A, Chen YC, Panula P, Rauvala H (2011) High mobility group box-1 (HMGB1; amphoterin) is required for zebrafish brain development. J Biol Chem 286:23200–23213
Guo J, Duckles SP, Weiss JH, Li X, Krause DN (2012) 17beta-Estradiol prevents cell death and mitochondrial dysfunction by an estrogen receptor-dependent mechanism in astrocytes after oxygen-glucose deprivation/reperfusion. Free Radic Biol Med 52:2151–2160
Louis SA, Mak CK, Reynolds BA (2013) Methods to culture, differentiate, and characterize neural stem cells from the adult and embryonic mouse central nervous system. Methods Mol Biol 946:479–506
Meneghini V, Francese MT, Carraro L, Grilli M (2010) A novel role for the receptor for advanced glycation end-products in neural progenitor cells derived from adult subventricular zone. Mol Cell Neurosci 45:139–150
Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK (2006) HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci 26:6413–6421
Chen X, Tian Y, Yao L, Zhang J, Liu Y (2010) Hypoxia stimulates proliferation of rat neural stem cells with influence on the expression of cyclin D1 and c-Jun N-terminal protein kinase signaling pathway in vitro. Neuroscience 165:705–714
Yang C, Rahimpour S, Yu AC, Lonser RR, Zhuang Z (2013) Regulation and dysregulation of astrocyte activation and implications in tumor formation. Cell Mol Life Sci 70:4201–4211
Pekny M, Nilsson M (2005) Astrocyte activation and reactive gliosis. Glia 50:427–434
Bao Y, Qin L, Kim E, Bhosle S, Guo H, Febbraio M, Haskew-Layton RE, Ratan R, Cho S (2012) CD36 is involved in astrocyte activation and astroglial scar formation. J Cereb Blood Flow Metab 32:1567–1577
Jeong SR, Kwon MJ, Lee HG, Joe EH, Lee JH, Kim SS, Suh-Kim H, Kim BG (2012) Hepatocyte growth factor reduces astrocytic scar formation and promotes axonal growth beyond glial scars after spinal cord injury. Exp Neurol 233:312–322
Kawano H, Kimura-Kuroda J, Komuta Y, Yoshioka N, Li HP, Kawamura K, Li Y, Raisman G (2012) Role of the lesion scar in the response to damage and repair of the central nervous system. Cell Tissue Res 349:169–180
Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, Bethea JR (2014) Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia 62:452–467
Lau LT, Yu AC (2001) Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma 18:351–359
Hayakawa K, Arai K, Lo EH (2010) Role of ERK map kinase and CRM1 in IL-1beta-stimulated release of HMGB1 from cortical astrocytes. Glia 58:1007–1015
Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T, Nishibori M (2007) Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J 21:3904–3916
Agnello D, Wang H, Yang H, Tracey KJ, Ghezzi P (2002) HMGB-1, a DNA-binding protein with cytokine activity, induces brain TNF and IL-6 production, and mediates anorexia and taste aversion. Cytokine 18:231–236
Liu H, Yao YM, Ding LH, Zhang H, Yuan B, Song Q, Ye QN, Huang CF, Sheng ZY (2009) High mobility group box-1 protein acts as a coactivator of nuclear factor of activated T cells-2 in promoting interleukin-2 transcription. Int J Biochem Cell Biol 41:641–648
Kim JB, Lim CM, Yu YM, Lee JK (2008) Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J Neurosci Res 86:1125–1131
Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA (2008) Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab 28:927–938
Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M (2008) The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci 28:12023–12031
Hayakawa K, Nakano T, Irie K, Higuchi S, Fujioka M, Orito K, Iwasaki K, Jin G, Lo EH, Mishima K, Fujiwara M (2010) Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. J Cereb Blood Flow Metab 30:871–882
Lei C, Lin S, Zhang C, Tao W, Dong W, Hao Z, Liu M, Wu B (2013) Effects of high-mobility group box1 on cerebral angiogenesis and neurogenesis after intracerebral hemorrhage. Neuroscience 229:12–19
Gabryel B, Bielecka A, Bernacki J, Labuzek K, Herman ZS (2011) Immunosuppressant cytoprotection correlates with HMGB1 suppression in primary astrocyte cultures exposed to combined oxygen-glucose deprivation. Pharmacol Rep 63:392–402
Yoshida H, Mimura J, Imaizumi T, Matsumiya T, Ishikawa A, Metoki N, Tanji K, Ota K, Hayakari R, Kosaka K, Itoh K, Satoh K (2011) Edaravone and carnosic acid synergistically enhance the expression of nerve growth factor in human astrocytes under hypoxia/reoxygenation. Neurosci Res 69:291–298
Koyama Y, Maebara Y, Hayashi M, Nagae R, Tokuyama S, Michinaga S (2012) Endothelins reciprocally regulate VEGF-A and angiopoietin-1 production in cultured rat astrocytes: implications on astrocytic proliferation. Glia 60:1954–1963
Kuric E, Wieloch T, Ruscher K (2013) Dopamine receptor activation increases glial cell line-derived neurotrophic factor in experimental stroke. Exp Neurol 247:202–208
Modi KK, Sendtner M, Pahan K (2013) Up-regulation of Ciliary Neurotrophic Factor in Astrocytes by Aspirin: implications for remyelination in multiple sclerosis. J Biol Chem 288:18533–18545
Sung SM, Jung DS, Kwon CH, Park JY, Kang SK, Kim YK (2007) Hypoxia/reoxygenation stimulates proliferation through PKC-dependent activation of ERK and Akt in mouse neural progenitor cells. Neurochem Res 32:1932–1939
Kim JH, Nam SW, Kim BW, Choi W, Lee JH, Kim WJ, Choi YH (2010) Astaxanthin Improves Stem Cell Potency via an Increase in the Proliferation of Neural Progenitor Cells. Int J Mol Sci 11:5109–5119
Yang J, Chen L, Ding J, Rong H, Dong W, Li X (2012) High mobility group box-1 induces migration of vascular smooth muscle cells via TLR4-dependent PI3K/Akt pathway activation. Mol Biol Rep 39:3361–3367
Lao CL, Lu CS, Chen JC (2013) Dopamine D3 receptor activation promotes neural stem/progenitor cell proliferation through AKT and ERK1/2 pathways and expands type-B and -C cells in adult subventricular zone. Glia 61:475–489
Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI (2011) Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 8:59–71
Zhang Q, Liu G, Wu Y, Sha H, Zhang P, Jia J (2011) BDNF promotes EGF-induced proliferation and migration of human fetal neural stem/progenitor cells via the PI3K/Akt pathway. Molecules 16:10146–10156
Peltier J, O’Neill A, Schaffer DV (2007) PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol 67:1348–1361
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 30470606) and the Program of the Traditional Chinese Medical Research of Chongqing Municipal Health Bureau (No. 2003-B-16).
Conflict of interest
All authors declare that they have no perceived or actual conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, M., Sun, L., Li, Y. et al. Oxygen Glucose Deprivation/Reperfusion Astrocytes Promotes Primary Neural Stem/Progenitor Cell Proliferation by Releasing High-Mobility Group Box 1. Neurochem Res 39, 1440–1450 (2014). https://doi.org/10.1007/s11064-014-1333-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1333-z