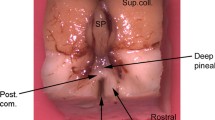
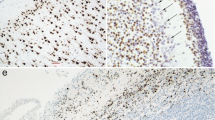
Objective. To study the cellular composition of the human epiphysis. Materials and methods. An immunohistochemical method using cell-specific markers selectively detecting astroglial, endothelial, nerve, and mast cells was employed to study the epiphysis in seven males aged 16–68 years. Antibodies to glial fibrillary acidic protein (GFAP), vimentin, low molecular weight neurofilament proteins (clone 2F11), and mast cell tryptase were used. Results. Immunohistochemical reactions for GFAP in the human epiphysis identified a large number of astroglial processes but few astroglial cell bodies. Cells had relatively small primary processes which were significantly thicker than those of stellate astrocytes in other parts of the brain. Astrocyte processes formed dense plexuses around blood vessels and many concretions. Many cell processes in the stroma and particularly the parenchyma and blood vessel endothelial cells were vimentin-immunoreactive. Coexpression of GFAP and vimentin was not seen in any of the structures studied. Low molecular weight neurofilament proteins were detected in occasional pinealocytes and their processes. Mast cells immunohistochemically labeled for tryptase were detected in all the epiphyseal formations studied (most frequently in the stroma) Conclusions: 1) GFAP-immunopositive astrocytes in the human epiphysis differed in terms of their morphological features from typical stellate astrocytes in other parts of the brain, such that pineal GFAP-immunopositive astrocytes could be placed in a separate subgroup of astrocytes; 2) human epiphyseal astrocytes did not simultaneously contain GFAP and vimentin, in contrast to pineal astrocytes in other mammals; 3) mast cells are a consistent component of the human epiphysis – obligatory in the stroma and facultative in the parenchyma; 4) human epiphyseal pinealocytes express neuron-specific neurofilament protein, which is evidence supporting their neuron-like nature; 5) the localization of neuron-like endocrine cells and significant quantities of immunocompetent mast cells in the human epiphysis define this endocrine organ as an important component of the unified neuroimmunoendocrine system of the body.
Similar content being viewed by others
References
I. P. Grigorev, O. S. Alekseeva, O. V. Kirik, et al., “Distribution of low molecular weight neurofi lament proteins in the cingulate cortex of the brain in rats,” Morfologiya, 154, No. 5, 7–12 (2018).
E. A. Fedorova, I. P. Grigorev, M. A. Syrtsova, et al., “Detection of the morphological signs of degranulation of human brain vascular plexus mast cells using various staining and immunohistochemical methods,” Morfologiya, 153, No. 2, 70–75 (2018).
E. A. Fedorova, D. A. Sufieva, I. P. Grigorev, and D. E. Korzhevskii, “Mast cells in the human epiphysis,” Usp. Gerontol., 31, No. 4, 484–489 (2018), https://doi.org/10.1134/S2079057019010053.
V. Kh. Khavinson, I. M. Kvetnoi, V. V. Popuchiev, et al., “Effects of pineal gland peptides on neuroendocrine interactions after pinealectomy,” Arkh. Patol., 63, No. 3, 18–21 (2001).
A. Borregón, J. Boya, J. L. Calvo, and F. López-Muñoz, “Immunohistochemical study of the pineal glial cells in the postnatal development of the rat pineal gland,” J. Pineal Res., 14, No. 2, 78–83 (1993), https://doi.org/10.1111/j.1600-079X.1993.tb00489.x.
S. S. Erlich and M. L. Apuzzo, “The pineal gland: anatomy, physiology, and clinical signifi cance,” J. Neurosurg., 63, No. 3, 321–341 (1985), https://doi.org/10.3171/jns.1985.63.3.0321.
A. Jouvet, M. Fevre-Montange, R. Besancon, et al., “Structural and ultrastructural characteristics of human pineal gland, and pineal parenchymal tumors,” Acta Neuropathol., 88, No. 4, 334–348 (1994), https://doi.org/10.1007/BF00310377.
F. Lopez-Munoz, J. L. Calvo, J. Boya, and A. L. Carboneil, “Coexpression of vimentin and glial fi brillary acidic protein in glial cells of the adult rat pineal gland,” J. Pineal Res., 12, No. 4, 145–148 (1992), https://doi.org/10.1111/j.1600-079x.1992.tb00041.x.
M. M. Macchi and J. N. Bruce, “Human pineal physiology and functional signifi cance of melatonin,” Front. Neuroendocrinol., 25, No. 3–4, 177–195 (2004), https://doi.org/10.1016/j.yfrne.2004.08.001.
M. Møller and F. M. Baeres, “The anatomy and innervation of the mammalian pineal gland,” Cell Tissue Res., 309, No. 1, 139–150 (2002), https://doi.org/10.1007/s00441-002-0580-5.
N. A. Oberheim, T. Takano, X. Han, et al., “Uniquely hominid features of adult human astrocytes,” J. Neurosci., 29, No. 10, 3276–3287 (2009), https://doi.org/10.1523/JNEUROSCI.4707-08.2009.
P. Redecker, Y. Cetin, neuron H.-W. Korf, “Differential immunocytochemical localization of calretinin in the pineal gland of three mammalian species,” J. Neurocytol., 25, No. 1, 9–18 (1996), https://doi.org/10.1007/bf02284782.
R. J. Reiter, D. X. Tan, S. J. Kim, and M. H. C. Cruz, “Delivery of pineal melatonin to the brain and SCN: role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces,” Brain Struct. Funct., 219, No. 6, 1873–1887 (2014), https://doi.org/10.1007/s00429-014-0719-7.
R. M. Slominski, R. J. Reiter, N. Schlabritz-Loutsevitch, et al., “Melatonin membrane receptors in peripheral tissues: distribution and functions,” Mol. Cell Endocrinol., 351, No. 2, 152–166 (2012), https://doi.org/10.1016/j.mce.2012.01.004.
X. Zang, G. Nilaver, B. M. Stein, et al., “Immunocytochemistry of pineal astrocytes: species differences and functional implications,” J. Neuropathol. Exp. Neurol., 44, No. 5, 486–495 (1985), https://doi.org/10.1097/00005072-198509000-00004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Morfologiya, Vol. 158, Nos. 4–5, pp. 19–26, July–October, 2020.
Rights and permissions
About this article
Cite this article
Grigorev, I.P., Fedorova, E.A., Sufieva, D.A. et al. Immunohistochemical Studies of Cell Organization in the Human Epiphysis. Neurosci Behav Physi 51, 546–552 (2021). https://doi.org/10.1007/s11055-021-01103-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-021-01103-4