Abstract
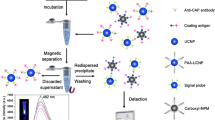
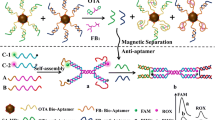
Ochratoxin A (OTA), the most harmful and abundant ochratoxin, is chemically stable and commonly existed in foodstuffs. In this work, upconversion luminescent-magnetic microbeads (UCLMMs) -based cytometric bead array for OTA detection with a less reagent consumption and high sensitivity has been established and optimized. In UCLMMs, upconversion nanocrystals (UCNs) for optical code present a weak background noise and no spectral cross talk between the encoding signals and target labels under two excitation conditions to improve detection sensitivity. While the superparamagnetic Fe3O4 nanoparticles (Fe3O4 NPs) aim for rapid analysis. The results show that the developed method has a sensitivity of 9.553 ppt below HPLC with a 50-μL sample and can be completed in <2 h with good accuracy and high reproducibility. Therefore, different colors of UCLMMs will become a promising assay platform for multiple mycotoxins after further improvement.

Graphical abstract








Similar content being viewed by others
References
Birtwell SW, Morgan H (2009) Microparticle encoding technologies for high-throughput multiplexed suspension assays. Integr Biol 1:345–362
Bonetta L (2005) Flow cytometry smaller and better. Nat Meth 2:785–795
Chen J, Fang Z, Liu J, Zeng L (2012) A simple and rapid biosensor for ochratoxin A based on a structure-switching signaling aptamer. Food Control 25:555–560. doi:10.1016/j.foodcont.2011.11.039
Creppy EE (2002) Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol Lett 127:19–28. doi:10.1016/S0378-4274(01)00479-9
Dasso J, Lee J, Bach H, Mage RG (2002) A comparison of ELISA and flow microsphere-based assays for quantification of immunoglobulins. J Immunol Methods 263:23–33. doi:10.1016/S0022-1759(02)00028-5
Duarte SC, Lino CM, Pena A (2010) Mycotoxin food and feed regulation and the specific case of ochratoxin A: a review of the worldwide status. Food Additives & Contaminants: Part A 27:1440–1450. doi:10.1080/19440049.2010.497166
Flajs D, Domijan AM, Ivić D, Cvjetković B, Peraica M (2009) ELISA and HPLC analysis of ochratoxin A in red wines of Croatia. Food Control 20:590–592. doi:10.1016/j.foodcont.2008.08.021
Gorris HH, Wolfbeis OS (2013) Photon-upconverting nanoparticles for optical encoding and multiplexing of cells. Biomolecules, and Microspheres Angewandte Chemie 52:3584
Guo X et al (2014) Development of an ultrasensitive aptasensor for the detection of aflatoxin B 1. Biosensors & Bioelectronics 56C:340–344
Huang K, Idris NM, Zhang Y (2015) Engineering of lanthanide-doped upconversion nanoparticles for optical encoding. Small 12:836
Hussein HS, Brasel JM (2001) Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 167:101–134. doi:10.1016/S0300-483X(01)00471-1
Kellar KL, Iannone MA (2002) Multiplexed microsphere-based flow cytometric assays. Exp Hematol 30:1227–1237. doi:10.1016/S0301-472X(02)00922-0
Lee J, Bisso P, Srinivas RL, Kim JJ, Swiston A, Doyle PS (2014) Universal process-inert encoding architecture for polymer microparticles. Nat Mater 13:524–529
Li Y, Wu H, Guo L, Zheng Y, Guo Y (2012) Microsphere-based flow cytometric immunoassay for the determination of citrinin in red yeast rice. Food Chem 134:2540–2545. doi:10.1016/j.foodchem.2012.04.072
Müller G et al (2003) Effects of the mycotoxin ochratoxin A and some of its metabolites on the human cell line THP-1. Toxicology 184:69–82. doi:10.1016/S0300-483X(02)00593-0
Malhotra BD, Srivastava S, Ali MA, Singh C (2014) Nanomaterial-based biosensors for food toxin detection Applied Biochemistry and Biotechnology. Applied Biochemistry and Biotechnology 174:880–896. doi:10.1007/s12010-014-0993-0
Mao J, Lei S, Yang X, Xiao D (2013) Quantification of ochratoxin A in red wines by conventional HPLC–FLD using a column packed with core–shell particles. Food Control 32:505–511
Novo P, Moulas G, França Prazeres DM, Chu V, Conde JP (2013) Detection of ochratoxin A in wine and beer by chemiluminescence-based ELISA in microfluidics with integrated photodiodes. Sensors Actuators B Chem 176:232–240. doi:10.1016/j.snb.2012.10.038
Pena A, Cerejo F, Lino C, Silveira I (2005) Determination of ochratoxin A in Portuguese rice samples by high performance liquid chromatography with fluorescence detection. Anal Bioanal Chem 382:1288–1293. doi:10.1007/s00216-005-3254-9
Pfohl-Leszkowicz A, Manderville RA (2007) Ochratoxin A: an overview on toxicity and carcinogenicity in animals and humans. Mol Nutr Food Res 51:61–99. doi:10.1002/mnfr.200600137
Pfohl-Leszkowicz A, Manderville RA (2012) An update on direct genotoxicity as a molecular mechanism of ochratoxin A carcinogenicity. Chem Res Toxicol 25:252–262. doi:10.1021/tx200430f
Ren W et al (2013) A cytometric bead assay for sensitive DNA detection based on enzyme-free signal amplification of hybridization chain reaction. Biosens Bioelectron 49:380–386. doi:10.1016/j.bios.2013.05.055
Rivas L, Mayorga-Martinez CC, Quesada-González D, Zamora-Gálvez A, de la Escosura-Muñiz A, Merkoçi A (2015) Label-free impedimetric aptasensor for ochratoxin-A detection using iridium oxide nanoparticles. Anal Chem 87:5167–5172. doi:10.1021/acs.analchem.5b00890
Sathe TR, Agrawal A, Nie S (2006) Mesoporous silica beads embedded with semiconductor quantum dots and iron oxide nanocrystals: dual-function microcarriers for optical encoding and magnetic separation. Anal Chem 78:5627–5632. doi:10.1021/ac0610309
Soleimany F, Jinap S, Faridah A, Khatib A (2012) A UPLC–MS/MS for simultaneous determination of aflatoxins, ochratoxin A, zearalenone, DON, fumonisins, T-2 toxin and HT-2 toxin, in cereals. Food Control 25:647–653. doi:10.1016/j.foodcont.2011.11.012
Solfrizzo M, Gambacorta L, Lattanzio VMT, Powers S, Visconti A (2011) Simultaneous LC–MS/MS determination of aflatoxin M1, ochratoxin A, deoxynivalenol, de-epoxydeoxynivalenol, α and β-zearalenols and fumonisin B1 in urine as a multi-biomarker method to assess exposure to mycotoxins. Anal Bioanal Chem 401:2831–2841. doi:10.1007/s00216-011-5354-z
Song T et al (2014) Self-healing encapsulation strategy for preparing highly stable, functionalized quantum-dot barcodes. ACS Applied Materials & Interfaces 6:2745–2752. doi:10.1021/am405285u
Song T et al (2011) Structural design and preparation of high-performance QD-encoded polymer beads for suspension arrays. J Mater Chem 21:2169–2177. doi:10.1039/C0JM02447C
Studer-Rohr I, Schlatter J, Dietrich RD (2000) Kinetic parameters and intraindividual fluctuations of ochratoxin A plasma levels in humans. Arch Toxicol 74:499–510. doi:10.1007/s002040000157
Sun AL, Zhang Y, Sun GP, Wang X, Tang D (2015) Homogeneous electrochemical detection of ochratoxin A in foodstuff using aptamer-graphene oxide nanosheets and DNase I-based target recycling reaction Biosensors and Bioelectronics
Tsai HY, Gao BZ, Yang SF, Li CS, Fuh CB (2013) Detection of alpha-fetoprotein in magnetic immunoassay of thin channels using biofunctional nanoparticles. J Nanopart Res 16:1–8. doi:10.1007/s11051-013-2182-4
Wang B, Wu Y, Chen Y, Weng B, Xu L, Li C (2016) A highly sensitive aptasensor for OTA detection based on hybridization chain reaction and fluorescent perylene probe. Biosens Bioelectron 81:125–130
Wang H-Q, Liu T-C, Cao Y-C, Huang Z-L, Wang J-H, Li X-Q, Zhao Y-D (2006) A flow cytometric assay technology based on quantum dots-encoded beads. Anal Chim Acta 580:18–23. doi:10.1016/j.aca.2006.07.048
Wu S, Duan N, Zhu C, Ma X, Wang M, Wang Z (2011) Magnetic nanobead-based immunoassay for the simultaneous detection of aflatoxin B1 and ochratoxin A using upconversion nanoparticles as multicolor labels Biosens Bioelectron 30:35–42 doi: 10.1016/j.bios.2011.08.023
Xi D, Luo X, Lu Q, Yao K, Liu Z, Ning Q (2008) The detection of HBV DNA with gold-coated iron oxide nanoparticle gene probes. J Nanopart Res 10:393–400. doi:10.1007/s11051-007-9263-1
Yang J, Gao P, Liu Y, Li R, Ma H, Du B, Wei Q (2015) Label-free photoelectrochemical immunosensor for sensitive detection of ochratoxin A. Biosensors and Bioelectronics 64:13–18. doi:10.1016/j.bios.2014.08.025
Yue S et al (2014) Simultaneous detection of ochratoxin A and fumonisin B1 in cereal samples using an aptamer-photonic crystal encoded suspension array. Anal Chem 86:11797–11802
Zhang L, Dong W-F, Sun H-B (2013) Multifunctional superparamagnetic iron oxide nanoparticles: design, synthesis and biomedical photonic applications. Nanoscale 5:7664–7684. doi:10.1039/C3NR01616A
Zhang L et al (2012) Magnetic/upconversion luminescent mesoparticles of Fe3O4@LaF3:Yb3+, Er3+ for dual-modal bioimaging. Chem Commun 48:11238–11240. doi:10.1039/C2CC36059D
Zhang Y et al (2016) Multifunctional microspheres encoded with upconverting nanocrystals and magnetic nanoparticles for rapid separation and immunoassays. ACS Appl Mater Interfaces 8:745–753. doi:10.1021/acsami.5b09913
Zhu Z et al (2015) An aptamer based surface plasmon resonance biosensor for the detection of ochratoxin A in wine and peanut oil. Biosens Bioelectron 65:320–326
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31401578, 51373117, 51303126, 51573128, and 81402575) and Tianjin Natural Science Foundation (13JCZDJC33200 and 15JCQNJC03100).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Zhenyu Liao, Ying Zhang, Lin Su, Jin Chang, and Hanjie Wang declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects.
Informed consent
Not applicable.
Additional information
Zhenyu Liao and Ying Zhang contributed equally to this work.
Electronic supplementary material
ESM 1
(PDF 490 kb)
Rights and permissions
About this article
Cite this article
Liao, Z., Zhang, Y., Su, L. et al. Application of upconversion luminescent-magnetic microbeads with weak background noise and facile separation in ochratoxin A detection. J Nanopart Res 19, 60 (2017). https://doi.org/10.1007/s11051-016-3717-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3717-2