Abstract
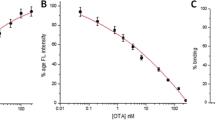
A magnetic beads (MBs)–assisted fluorescence aptasensing approach based on dual DNA tweezers and magnetic separation was established for the detection of ochratoxin A (OTA) and fumonisin B1 (FB1). A dual DNA tweezers structure with four ends linked with fluorophores (FAM, ROX) and quenchers (BHQ1, BHQ2) was designed, and produced the high initial fluorescence signals because of the long spatial distance between FAM and BHQ1, ROX, and BHQ2. Bio-aptamer/anti-aptamer of OTA and bio-aptamer/anti-aptamer of FB1 were respectively annealed to form dsDNA, and immobilized to MBs coated with streptavidin (SA). With the existence of OTA and FB1, OTA and FB1 preferentially bound with their respective bio-aptamers, which made anti-aptamers dissociate from dsDNA coupled on MBs. After magnetic separation, the dissociated anti-aptamers reacted with dual DNA tweezers, respectively, which made DNA tweezers close and the fluorescence was quenched. The linear ranges of approach for OTA and FB1 detection were 0.05–20 ng/mL and 0.1–40 ng/mL, respectively. The limit of detection for OTA and FB1 was 0.029 ng/mL and 0.061 ng/mL. The prepared MBs-assisted fluorescence aptasensing approach was applied to detect OTA and FB1 in spiked red wine and corn samples, which showed good recoveries between 92 and 106%.






Similar content being viewed by others
References
Zhang LL, Zhang ZY, Tian Y, Cui MH, Huang BB, Luo T, Zhang SF, Wang HJ. Rapid, simultaneous detection of mycotoxins with smartphone recognition-based immune microspheres. Anal Bioanal Chem. 2021;413:3683–93. https://doi.org/10.1007/s00216-021-03316-5.
Yang MY, Cui MH, Wang WX, Yang YD, Chang J, Hao JY, Wang HJ. Background-free upconversion-encoded microspheres for mycotoxin detection based on a rapid visualization method. Anal Bioanal Chem. 2020;412:81–91. https://doi.org/10.1007/s00216-019-02206-1.
Alkadri D, Rubert J, Prodi A, Pisi A, Manes J, Soler C. Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chem. 2014;157:111–8. https://doi.org/10.1016/j.foodchem.2014.01.052.
Yang X, Gao J, Liu Q, Yang DJ. Co-occurrence of mycotoxins in maize and maize-derived food in China and estimation of dietary intake. Food Addit Contam B. 2019;12:124–34. https://doi.org/10.1080/19393210.2019.1570976.
Oplatowska-Stachowiak M, Haughey SA, Chevallier OP, Galvin-King P, Campbell K, Magowan E, Adam G, Berthiller F, Krska R, Elliott CT. Determination of the mycotoxin content in distiller’s dried frain with solubles using a multianalyte UHPLC-MS/MS method. J Agric Food Chem. 2015;63:9441–51. https://doi.org/10.1021/acs.jafc.5b03844.
Klaric MK, Rumora L, Ljubanovic D, Pepeljnjak S. Cytotoxicity and apoptosis induced by fumonisin B-1, beauvericin and ochratoxin A in porcine kidney PK15 cells: effects of individual and combined treatment. Arch Toxicol. 2008;82:247–55. https://doi.org/10.1007/s00204-007-0245-y.
Creppy EE, Chiarappa P, Baudrimont P, Moukha S, Carratu MR. Synergistic effects of fumonisin B-1 and ochratoxin A: are in vitro cytotoxicity data predictive of in vivo acute toxicity? Toxicol. 2004;201:115–23. https://doi.org/10.1016/j.tox.2004.04.008.
Mwanza M, Kametler L, Bonai A, Rajli V, Kovacs M, Dutton MF. The cytotoxic effect of fumonisin B1 and ochratoxin A on human and pig lymphocytes using the methyl thiazol tetrazolium (MTT) assay. Mycotoxin Res. 2009;25:233–8. https://doi.org/10.1007/s12550-009-0033-z.
Wang HY, Wei YJ, Xie Y, Yan C, Du HZ, Li ZN. Ochratoxin A and fumonisin B-1 exhibit synergistic cytotoxic effects by inducing apoptosis on rat liver cells. Toxicon. 2020;181:19–27. https://doi.org/10.1016/j.toxicon.2020.04.094.
Lee HJ, Ryu D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: public health perspectives of their co-occurrence. J Agric Food Chem. 2017;65:7034–51. https://doi.org/10.1021/acs.jafc.6b04847.
Pellicer-Castell E, Belenguer-Sapina C, Borras VJ, Amoros P, El Haskouri J, Herrero-Martinez JM, Mauri-Aucejo AR. Extraction of aflatoxins by using mesoporous silica (type UVM-7), and their quantitation by HPLC-MS. Microchim Acta. 2019;186:792. https://doi.org/10.1007/s00604-019-3958-8.
Stadler D, Sulyok M, Schuhmacher R, Berthiller F, Krska R. The contribution of lot-to-lot variation to the measurement uncertainty of an LC-MS-based multi-mycotoxin assay. Anal Bioanal Chem. 2018;410:4409–18. https://doi.org/10.1007/s00216-018-1096-5.
Qie ZW, Yan WL, Gao ZC, Meng W, Xiao R, Wang SQ. An anti-BSA antibody-based immunochromatographic assay for chloramphenicol and aflatoxin M-1 by using carboxy-modified CdSe/ZnS core-shell nanoparticles as label. Microchim Acta. 2020;187:10. https://doi.org/10.1007/s00604-019-4009-1.
Ediage EN, Mavungu JDD, Goryacheva IY, Peteghem CV, Saeger SD. Multiplex flow-through immunoassay formats for screening of mycotoxins in a variety of food matrices. Anal Bioanal Chem. 2012;403:265–78. https://doi.org/10.1007/s00216-012-5803-3.
Lin BX, Yu Y, Cao YJ, Guo ML, Zhu DB, Dai JX, Zheng MS. Point-of-care testing for streptomycin based on aptamer recognizing and digital image colorimetry by smartphone. Biosens Bioelectron. 2018;100:482–9. https://doi.org/10.1016/j.bios.2017.09.028.
Bhardwaj H, Marquette CA, Dutta P, Rajesh SG. Integrated graphene quantum dot decorated functionalized nanosheet biosensor for mycotoxin detection. Anal Bioanal Chem. 2020;412:7029–41. https://doi.org/10.1007/s00216-020-02840-0.
Bonel L, Vidal JC, Duato P, Castillo JR. An electrochemical competitive biosensor for ochratoxin A based on a DNA biotinylated aptamer. Biosens Bioelectron. 2011;26:3254–9. https://doi.org/10.1016/j.bios.2010.12.036.
He DY, Wu ZZ, Cui B, Jin ZY, Xu EB. A fluorometric method for aptamer based simultaneous determination of two kinds of the fusarium mycotoxins zearalenone and fumonisin B-1 making use of gold nanorods and upconversion nanoparticles. Microchim Acta. 2020;187:254. https://doi.org/10.1007/s00604-020-04236-4.
He DY, Wu ZZ, Cui B, Xu EB. Aptamer and gold nanorod-based fumonisin B1 assay using both fluorometry and SERS. Microchim Acta. 2020;187:215. https://doi.org/10.1007/s00604-020-4192-0.
Shi JR, Li GY, Cui YR, Zhang Y, Liu DH, Shi Y, He H. Surface-imprinted beta-cyclodextrin-functionalized carbon nitride nanosheets for fluorometric determination of sterigmatomycin. Microchim Acta. 2020;186:808. https://doi.org/10.1007/s00604-019-3867-x.
Taghdisi SM, Danesh NM, Beheshti HR, Ramezani M, Abnous K. A novel fluorescent aptasensor based on gold and silica nanoparticles for the ultrasensitive detection of ochratoxin A. Nanoscale. 2016;8:3439–46. https://doi.org/10.1039/c5nr08234j.
Maragos CM. Recent advances in the development of novel materials for mycotoxin analysis. Anal Bioanal Chem. 2009;395:1205–13. https://doi.org/10.1007/s00216-009-2728-6.
Li L, Chen HP, Lv XL, Wang M, Jiang XZ, Jiang YF, Wang HY, Zhao YF, Xia LR. Paper-based immune-affinity arrays for detection of multiple mycotoxins in cereals. Anal Bioanal Chem. 2018;410:2253–62. https://doi.org/10.1007/s00216-018-0895-z.
Jiang LP, Peng JD, Yuan R, Chai YQ, Yuan YL, Bai LJ, Wang Y. An aptasensing platform for simultaneous detection of multiple analytes based on the amplification of exonuclease-catalyzed target recycling and DNA concatemers. Analyst. 2013;138:4818–22. https://doi.org/10.1039/c3an00757j.
Xiong ZW, Wang Q, Xie YJ, Li N, Yun W, Yang LZ. Simultaneous detection of aflatoxin B1 and ochratoxin A in food samples by dual DNA tweezers nanomachine. Food Chem. 2021;338:128122. https://doi.org/10.1016/j.foodchem.2020.128122.
Wu G, Xiong ZW, Oh SH, Ren YR, Wang Q, Yang LZ. Two-color, ultra-sensitive fluorescent strategy for ochratoxin A detection based on hybridization chain reaction and DNA tweezers. Food Chem. 2021;356:129663. https://doi.org/10.1016/j.foodchem.2021.129663.
Wu G, Li YT, Zhang JF, Yun W, Xiong ZW, Yang LZ. Simultaneous and ultra-sensitive detection of Cu2+ and Mg2+ in wine and beer based on dual DNA tweezers and entropy-driven three-dimensional DNA nanomachine. Food Chem. 2021;358:129835. https://doi.org/10.1016/j.foodchem.2021.129835.
Cruz-Aguado JA, Penner G. Determination of ochratoxin A with a DNA aptamer. J Agric Food Chem. 2008;56:10456–61. https://doi.org/10.1021/jf801957h.
Shi ZY, Zheng YT, Zhang HB, He CH, Wu WD, Zhang HB. DNA electrochemical aptasensor for detecting fumonisin B1 based on graphene and thionine nanocomposite. Electroanalysis. 2015;5:1097–103. https://doi.org/10.1002/elan.201400504.
McKeague M, Bradley CR, Girolamo AD, Visconti A, Miller JD, Derosa MC. Screening and initial binding assessment of fumonisin B1 aptamers. Int J Mol Sci. 2010;11:4864–81. https://doi.org/10.3390/ijms11124864.
Zhao Y, Liu R, Sun W, Lv L, Guo Z. Ochratoxin A detection platform based on signal amplification by Exonuclease III and fluorescence quenching by gold nanoparticles. Sensors Actuators B Chem. 2018;255:1640–5. https://doi.org/10.1016/j.snb.2017.08.176.
Lv L, Li D, Cui C, Zhao Y, Guo Z. Nuclease-aided target recycling signal amplification strategy for ochratoxin. A monitoring. Biosens Bioelectron. 2017;87:136–41. https://doi.org/10.1016/j.bios.2016.08.024.
Oswald S, Karsunke X, Dietrich R, Martlbauer E, Niessner R, Knopp D. Automated regenerable microarray-based immunoassay for rapid parallel quantification of mycotoxins in cereals. Anal Bioanal Chem. 2013;405:6405–15. https://doi.org/10.1007/s00216-013-6920-3.
Hao N, Lu JW, Zhou Z, Hua R, Wang K. A pH-resolved colorimetric biosensor for simultaneous multiple target detection. ACS Sens. 2018;3:2159–65. https://doi.org/10.1021/acssensors.8b00717.
Wang CQ, Qian J, An KQ, Huang XY, Zhao LF, Liu Q, Hao N, Wang K. Magneto-controlled aptasensor for simultaneous electrochemical detection of dual mycotoxins in maize using metal sulfide quantum dots coated silica as labels. Biosens Bioelectron. 2017;89:802–9. https://doi.org/10.1016/j.bios.2016.10.010.
Wu SJ, Duan N, Ma XY, Xia Y, Wang HG, Wang ZP, Zhang Q. Multiplexed fluorescence resonance energy transfer aptasensor between upconversion nanoparticles and graphene oxide for the simultaneous determination of mycotoxins. Anal Chem. 2012;84:6263–70. https://doi.org/10.1021/ac301534w.
Molinero-Fernández Á, Moreno-Guzmán M, Ángel López MA, Escarpa A. Biosensing strategy for simultaneous and accurate quantitative analysis of mycotoxins in food samples using unmodified graphene micromotors. Anal Chem. 2017;89:10850–7. https://doi.org/10.1021/acs.analchem.7b02440.
Funding
This study was funded by the Key Scientific and Technological Project of Henan Province (212102310001) and the Innovative Funds Plan of Henan University of Technology (2020ZKCJ14).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qu, C., Zhao, L., He, X. et al. Magnetic beads–assisted fluorescence aptasensing approach based on dual DNA tweezers for detection of ochratoxin A and fumonisin B1 in wine and corn. Anal Bioanal Chem 413, 6677–6685 (2021). https://doi.org/10.1007/s00216-021-03635-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03635-7