Abstract
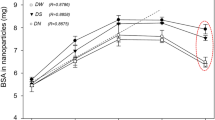
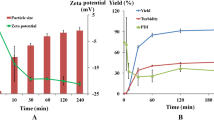
The desolvation method was successfully used to prepare nanoparticles from bovine serum albumin (BSA) using ethanol, acetone, and their mixtures (70:30 and 50:50, respectively). Ethanol and mixtures of ethanol and acetone led to the most spherical nanoparticles, while using pure acetone resulted in a mixture of spherical and rod shape nanoparticle. Acetone was the solvent with higher encapsulation efficiency equal to 99.2 ± 0.36 %. The polydispersity values of BSA NPs in this study were 0.045 ± 0.007, 0.065 ± 0.013, 0.091 ± 0.012, and 0.120 ± 0.016 for ethanol (100) 4×, Et:Ac (70:30) 4×, Et:Ac (50:50) 4×, and acetone (100) 3×, respectively. Encapsulation efficiencies of curcumin inside BSA NPs were 19.4 ± 2.2 and 19.8 ± 1.6 % for 1.0 and 1.5 molar ratios of curcumin to BSA, respectively. Crosslinking using glutaraldehyde improved the stability of BSA NPs and curcumin-loaded BSA NPs and both groups of nanoparticles were stable for 1 month; the lyophilized curcumin-loaded BSA NPs were able to redisperse in water. The particle size and polydispersity index of redispersed NPs were higher than the original NPs before lyophilization. The size distribution study shows that after 10 s of sonication most nanoparticles were well dispersed; however, a small but significant fraction formed aggregates. Sonication for 10 s decreased the effective diameter and polydispersity of the redispersed nanoparticles, while increasing the sonication time to 20 s did not show significant changes. In vitro release study of curcumin from BSA NPs showed that these biocompatible nanoparticles have the ability to be used as a carrier to improve controlled release of curcumin.









Similar content being viewed by others
References
Altunbas A, Lee SJ, Rajasekaran SA, Schneider JP, Pochan DJ (2011) Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 32(25):5906–5914
Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, Aggarwal BB (2010) Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol 79(3):330–338
Bourassa P, Kanakis CD, Tarantilis P, Pollissiou MG, Tajmir-Riahi HA (2010) Resveratrol, genistein, and curcumin bind bovine serum albumin. J Phys Chem B 114(9):3348–3354
Chin SF, Iyer KS, Saunders M, St Pierre TG, Buckley C, Paskevicius M, Raston CL (2009) Encapsulation and sustained release of curcumin using superparamagnetic silica reservoirs. Chem Eur J 15(23):5661–5665
Clark AH, Judge FJ, Richards JB, Stubbs JM, Suggett A (1981) Electron microscopy of network structures in thermally-induced globular protein gels. Int J Pept Prot Res 17(3):380–392
Das RK, Kasoju N, Bora U (2010) Encapsulation of curcumin in alginate–chitosan–pluronic composite nanoparticles for delivery to cancer cells. Nanomed Nanotechnol 6(1):e153–e160
Dhule SS, Penfornis P, Frazier T, Walker R, Feldman J, Tan G, He J, Alb A, John V, Pochampally R (2012) Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomed Nanotechnol 8(4):440–451
Duan J, Mansour HM, Zhang Y, Deng X, Chen Y, Wang J, Pan Y, Zhao J (2012) Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles. Int J Pharm 426(1–2):193–201
Elzoghby AO, Samy WM, Elgindy NA (2012) Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release 157:168–182
Gomez-Estaca J, Balaguer MP, Gavara R, Hernandez-Munoz P (2012) Formation of zein nanoparticles by electrohydrodynamic atomization: effect of the main processing variables and suitability for encapsulating the food coloring and active ingredient curcumin. Food Hydrocolloid 28(1):82–91
Gülseren I, Fang Y, Corredig M (2012a) Zinc incorporation capacity of whey protein nanoparticles prepared with desolvation with ethanol. Food Chem 135(2):770–774
Gülseren T, Fang Y, Corredig M (2012b) Whey protein nanoparticles prepared with desolvation with ethanol: characterization, thermal stability and interfacial behavior. Food Hydrocolloid 29(2):258–264
Kim TH, Jiang HH, Youn YS, Park CW, Tak KK, Lee S, Kim H, Jon S, Chen X, Lee KC (2011) Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int J Pharm 403:285–291
Kinsella JE, Whitehead DM (1989) Proteins in whey: chemical, physical, and functional properties. Adv Food Nutr Res 33(C):343–438
Ko S, Gunasekaran S (2006) Preparation of sub-100-nm β-lactoglobulin (BLG) nanoparticles. J Microencapsul 23(8):887–898
Koley P, Pramanik A (2012) Multilayer vesicles, tubes, various porous structures and organo gels through the solvent-assisted self-assembly of two modified tripeptides and their different applications. Soft Matter 8(19):5364–5374
Kolluru LP, Rizvi SAA, D’Souza M, D’Souza MJ (2013) Formulation development of albumin based theragnostic nanoparticles as a potential delivery system for tumor targeting. J Drug Target 21(1):77–86
Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB (2007) Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-KB–regulated gene products. Cancer Res 67(8):3853–3861
Kunwar A, Barik A, Pandey R, Priyadarsini KI (2006) Transport of liposomal and albumin loaded curcumin to living cells: an absorption and fluorescence spectroscopic study. BBA-Gen Subj 1760(10):1513–1520
Langer K, Balthasar S, Vogel V, Dinauer N, Von Briesen H, Schubert D (2003) Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int J Pharm 257:169–180
Leung MHM, Colangelo H, Kee TW (2008) Encapsulation of curcumin in cationic micelles suppresses alkaline hydrolysis. Langmuir 24(11):5672–5675
Li L, Zhao X, Yang C, Hu H, Qiao M, Chen D (2011) Preparation and optimization of doxorubicin-loaded albumin nanoparticles using response surface methodology. Drug Dev Ind Pharm 37(10):1170–1180
Li C, Zhang D, Guo H, Hao L, Zheng D, Liu G, Shen J, Tian X, Zhang Q (2013) Preparation and characterization of galactosylated bovine serum albumin nanoparticles for liver-targeted delivery of oridonin. Int J Pharm 448(1):79–86
Lin W, Coombes AG, Davies MC, Davis SS, Illum L (1993) Preparation of sub-100 nm human serum albumin nanospheres using a pH-coacervation method. J Drug Target 1(3):237–243
Maghsoudi A, Shojaosadati SA, Vasheghani Farahani E (2008) 5-Fluorouracil-loaded BSA nanoparticles: formulation optimization and in vitro release study. AAPS Pharm Sci Technol 9(4):1092–1096
Maheshwari RK, Singh AK, Gaddipati J, Srimal RC (2006) Multiple biological activities of curcumin: a short review. Life Sci 78(18):2081–2087
Manju S, Sreenivasan K (2011) Hollow microcapsules built by layer by layer assembly for the encapsulation and sustained release of curcumin. Colloid Surf B 82:588–593
Merodio M, Arnedo A, Renedo MJ, Irache JM (2000) Ganciclovir-loaded albumin nanoparticles: characterization and in vitro release properties. Eur J Pharm Sci 12(3):251–259
Moghimi SM, Hunter AC, Murray JC (2001) Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev 53:283–318
Mukerjee A, Vishwanatha JK (2009) Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res 29:3867–3876
Narayanan NK, Nargi D, Randolph C, Narayanan BA (2009) Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer 125(1):1–8
Nguyen HH, Ko S (2010) Preparation of size-controlled BSA nanoparticles by intermittent addition of desolvating agent. IFMBE Proc 27:231–234
Podaralla S, Perumal O (2012) Influence of formulation factors on the preparation of zein nanoparticles. AAPS Pharm Sci Technol 13(3):919–927
Rahimnejad M, Najafpour G, Bakeri G (2012) Investigation and modeling effective parameters influencing the size of BSA protein nanoparticles as colloidal carrier. Colloid Surf A 412:96–100
Sadeghi R, Kalbasi A, Emam-jomeh Z, Razavi SH, Kokini J, Moosavi-Movahedi AA (2013) Biocompatible nanotubes as potential carrier for curcumin as a model bioactive compound. J Nanopart Res 15:1031. doi:10.1007/s11051-013-1931-8
Sahu A, Kasoju N, Bora U (2008) Fluorescence study of the curcumin-casein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules 9(10):2905–2912
Sahu A, Kasoju N, Goswami P, Bora U (2011) Encapsulation of curcumin in Pluronic block copolymer micelles for drug delivery applications. J Biomater Appl 25(6):619–639
Salvioli S, Sikora E, Cooper EL, Franceschi C (2007) Curcumin in cell death processes: a challenge for CAM of age-related pathologies. Evid-based Complement Altern 4(2):181–190
Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MNVR (2009) Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci 37(3–4):223–230
Skrt M, Benedik E, Podlipnik C, Ulrih NP (2012) Interactions of different polyphenols with bovine serum albumin using fluorescence quenching and molecular docking. Food Chem 135:2418–2424
Sun M, Gao Y, Guo C, Cao F, Song Z, Xi Y, Yu A, Zhai G (2010) Enhancement of transport of curcumin to brain in mice by poly(n-butylcyanoacrylate) nanoparticle. J Nanopart Res 12(8):3111–3122
Teng Z, Luo Y, Wang Q (2012) Nanoparticles synthesized from soy protein: preparation, characterization, and application for nutraceutical encapsulation. J Agric Food Chem 60(10):2712–2720
Wang Z, Leung MHM, Kee TW, English DS (2010) The role of charge in the surfactant-assisted stabilization of the natural product curcumin. Langmuir 26(8):5520–5526
Wang N, Guan Y, Yang L, Jia L, Wei X, Liu H, Guo C (2013) Magnetic nanoparticles (MNPs) covalently coated by PEO–PPO–PEO block copolymer for drug delivery. J Colloid Interface Sci 395(1):50–57
Weber C, Kreuter J, Langer K (2000) Desolvation process and surface characteristics of HSA-nanoparticles. Int J Pharm 196:197–200
Zhao D, Zhao X, Zu Y, Li J, Zhang Y, Jiang R, Zhang Z (2010) Preparation, characterization, and in vitro targeted delivery of folate-decorated paclitaxel-loaded bovine serum albumin nanoparticles. Int J Nanomed 5:669–677
Acknowledgments
The majority of the work in this paper was conducted at the University of Illinois. Rohollah Sadeghi came to the University of Illinois with a Fellowship from the Ministry of Science Research and Technology of Iran. The support of the research from USDA Hatch funds, the Materials Research Lab, and the use of the spectrophotometer in Dr. Bhalerao’s lab are gratefully acknowledged. The support of the University of Tehran, Iran National Science Foundation, Center of Excellence in Biothermodynamics, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadeghi, R., Moosavi-Movahedi, A.A., Emam-jomeh, Z. et al. The effect of different desolvating agents on BSA nanoparticle properties and encapsulation of curcumin. J Nanopart Res 16, 2565 (2014). https://doi.org/10.1007/s11051-014-2565-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2565-1