Abstract
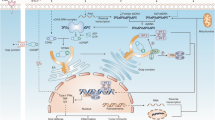
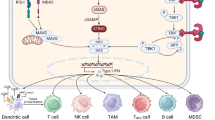
The stimulator of the interferon genes (STING) signaling pathway plays a crucial role in innate immunity by detecting cytoplasmic DNA and initiating antiviral host defense mechanisms. The STING cascade is triggered when the enzyme cyclic GMP-AMP synthase (cGAS) binds cytosolic DNA and synthesizes the secondary messenger cGAMP. cGAMP activates the endoplasmic reticulum adaptor STING, leading to the activation of kinases TBK1 and IRF3 that induce interferon production. Secreted interferons establish an antiviral state in infected and adjacent cells. Beyond infections, aberrant DNA in cancer cells can also activate the STING pathway. Preclinical studies have shown that pharmacological STING agonists like cyclic dinucleotides elicit antitumor immunity when administered intratumorally by provoking innate and adaptive immunity. Combining STING agonists with immune checkpoint inhibitors may improve outcomes by overcoming tumor immunosuppression. First-generation STING agonists encountered challenges like poor pharmacokinetics, limited tumor specificity, and systemic toxicity. The development of the next-generation STING-targeted drugs to realize the full potential of engaging this pathway for cancer treatment can be a solution to overcome the current challenges, but further studies are required to determine optimal applications and combination regimens for the clinic. Notably, the controlled activation of STING is needed to preclude adverse effects. This review explores the mechanisms and effects of STING activation, its role in cancer immunotherapy, and current challenges.




Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Islami F et al (2021) Annual report to the nation on the status of cancer, part 1: national cancer statistics JNCI: Journal of the National Cancer Institute, 113(12): p. 1648–1669
Siegel RL et al (2021) Cancer statistics, 2021. Ca Cancer J Clin 71(1):7–33
Duan Z, Luo Y (2021) Targeting macrophages in cancer immunotherapy. Signal Transduct Target Therapy 6(1):127
Hegde PS, Chen DS (2020) Top 10 challenges in cancer immunotherapy. Immunity 52(1):17–35
Zhang Y, Zhang Z (2020) The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol 17(8):807–821
Macciò A, Madeddu C (2023) Molecular Basis and Advances in Targeted Immunotherapy for Cancer. MDPI. p. 7802
Rallis KS et al (2021) Cytokine-based cancer immunotherapy: challenges and opportunities for IL-10. Anticancer Res 41(7):3247–3252
Ban W et al (2022) Emerging systemic delivery strategies of oncolytic viruses: a key step toward cancer immunotherapy. Nano Res 15(5):4137–4153
Taefehshokr S et al (2022) Cancer immunotherapy: challenges and limitations. Pathology-Research Pract 229:153723
Zarenezhad E et al (2023) Metallic nanoparticles: their potential role in breast Cancer immunotherapy via trained immunity provocation. Biomedicines, 11(5)
Bahmanyar M et al (2022) Opportunities and obstacles for the melanoma immunotherapy using T cell and chimeric antigen receptor T (CAR-T) applications: a literature review. Mol Biol Rep 49(11):10627–10633
Pauken KE et al (2019) Adverse events following cancer immunotherapy: obstacles and opportunities. Trends Immunol 40(6):511–523
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378(2):158–168
Cheng R, Santos HA (2023) Smart nanoparticle-based platforms for regulating Tumor Microenvironment and Cancer Immunotherapy. Adv Healthc Mater 12(8):2202063
Ventola CL (2017) Cancer immunotherapy, part 3: challenges and future trends. Pharm Ther 42(8):514
Patel A, Goldstein D, Tannock I (2022) Improving access to immunotherapy in low-and middle-income countries. Ann Oncol 33(4):360–361
Jiang M et al (2020) cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol 13:1–11
Reisländer T, Groelly FJ, Tarsounas M (2020) DNA damage and cancer immunotherapy: a STING in the tale. Mol Cell 80(1):21–28
Go E-J et al (2020) Combination of irreversible electroporation and STING agonist for effective cancer immunotherapy. Cancers 12(11):3123
Wu JJ et al (2020) Agonists and inhibitors of the STING pathway: potential agents for immunotherapy. Med Res Rev 40(3):1117–1141
Garland KM, Sheehy TL, Wilson JT (2022) Chemical and biomolecular strategies for STING pathway activation in cancer immunotherapy. Chem Rev 122(6):5977–6039
Su T et al (2022) Responsive multivesicular polymeric nanovaccines that codeliver STING agonists and neoantigens for combination tumor immunotherapy. Adv Sci 9(23):2201895
Yang Y et al (2021) ZBP1-MLKL necroptotic signaling potentiates radiation-induced antitumor immunity via intratumoral STING pathway activation. Sci Adv 7(41):eabf6290
Pan X et al (2023) Strategies involving STING pathway activation for cancer immunotherapy: mechanism and agonists. Biochem Pharmacol, : p. 115596
Ling YY et al (2022) Simultaneous photoactivation of cGAS-STING pathway and pyroptosis by platinum (II) triphenylamine complexes for Cancer Immunotherapy. Angew Chem Int Ed 61(43):e202210988
Zheng W et al (2023) How the Innate Immune DNA sensing cGAS-STING pathway is involved in apoptosis. Int J Mol Sci, 24(3)
Ou L et al (2021) The cGAS-STING pathway: a promising Immunotherapy Target. Front Immunol, 12
Decout A et al (2021) The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol 21(9):548–569
Liu N et al (2022) The cGAS-STING pathway in bacterial infection and bacterial immunity. Front Immunol 12:814709
Paulis A, Tramontano E (2023) Unlocking STING as a therapeutic antiviral strategy. Int J Mol Sci, 24(8)
Patel DJ, Yu Y, Xie W (2023) cGAMP-activated cGAS–STING signaling: its bacterial origins and evolutionary adaptation by metazoans. Nat Struct Mol Biol 30(3):245–260
Xia N et al (2020) African swine fever virus structural protein p17 inhibits cell proliferation through ER stress—ROS mediated cell cycle arrest. Viruses 13(1):21
Zhang R, Kang R, Tang D (2021) The STING1 network regulates autophagy and cell death. Signal Transduct Target Therapy 6(1):208
Heipertz EL, Harper J, Walker WE (2017) STING and TRIF contribute to mouse Sepsis, depending on severity of the Disease Model. Shock 47(5):621–631
Zhang R et al (2023) NLRC4 promotes the cGAS-STING signaling pathway by facilitating CBL-mediated K63-linked polyubiquitination of TBK1. J Med Virol 95(8):e29013
Tian M et al (2020) MYSM1 represses innate immunity and autoimmunity through suppressing the cGAS-STING pathway. Cell Rep, 33(3)
Song X et al (2023) The stimulator of interferon genes (STING) agonists for treating acute myeloid leukemia (AML): current knowledge and future outlook. Clin Transl Oncol 25(6):1545–1553
Yang C et al (2023) Role of the cGAS-STING pathway in radiotherapy for non-small cell lung cancer. Radiat Oncol 18(1):145
Gan Y et al (2021) The cGAS/STING pathway: a Novel Target for Cancer Therapy. Front Immunol 12:795401
Liao X-W, Zhang S-Y, Zhang X (2009) IFN-λ-newcomer to the interferon family
Ishikawa H, Barber GN (2008) STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455(7213):674–678
Hervas-Stubbs S et al (2011) Direct effects of type I interferons on cells of the immune system. Clin Cancer Res 17(9):2619–2627
Bolívar S et al (2018) IFN-β plays both pro-and anti-inflammatory roles in the rat cardiac fibroblast through differential STAT protein activation. Front Pharmacol 9:1368
Mantlo E et al (2020) Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res 179:104811
Daniel B, Stetson RM (2008) Type I interferons in host defense. Immunity 5(3):373–381
Schreiber G (2020) The role of type I interferons in the pathogenesis and treatment of COVID-19. Front Immunol 11:595739
Choi H, Shin EC (2021) Roles of type I and III interferons in COVID-19. Yonsei Med J 62(5):381–390
Kim Y-M, Shin E-C (2021) Type I and III interferon responses in SARS-CoV-2 infection. Exp Mol Med 53(5):750–760
King C, Sprent J (2021) Dual nature of type I interferons in SARS-CoV-2-induced inflammation. Trends Immunol 42(4):312–322
Latanova A, Starodubova E, Karpov V (2022) Flaviviridae nonstructural proteins: the role in Molecular mechanisms of triggering inflammation. Viruses, 14(8)
Kim D et al (2023) IFN-Induced protein with tetratricopeptide repeats 2 limits autoimmune inflammation by regulating myeloid cell activation and metabolic activity. J Immunol 210(6):721–731
Diaz-San Segundo F et al (2010) Interferon-induced protection against foot-and-mouth disease virus infection correlates with enhanced tissue-specific innate immune cell infiltration and interferon-stimulated gene expression. J Virol 84(4):2063–2077
Sher Y-P, Liu J-P, Liu S-J (2023) ADAM9 drives immunosuppressive environment for lung tumor progression. The Journal of Immunology
Kang SH et al (2019) Inhibition of MEK with trametinib enhances the efficacy of anti-PD-L1 inhibitor by regulating anti-tumor immunity in head and neck squamous cell carcinoma. Oncoimmunology 8(1):e1515057
Goetzke CC, Ebstein F, Kallinich T (2021) Role of proteasomes in inflammation. J Clin Med, 10(8)
Yang J et al (2022) Mechanism and effects of STING-IFN-I pathway on nociception: a narrative review. Front Mol Neurosci 15:1081288
Rojas M et al (2021) The Landscape of IFN/ISG Signaling in HIV-1-Infected macrophages and its possible role in the HIV-1 latency. Cells, 10(9)
Santiago KB et al (2023) Propolis anti-inflammatory effects on MAGE-1 and retinoic acid-treated dendritic cells and on Th1 and T regulatory cells. J Venom Anim Toxins Incl Trop Dis 29:e20220044
Sun S et al (2020) Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc Natl Acad Sci U S A 117(44):27509–27515
Phillips BE et al (2019) Tolerogenic dendritic cells and T-Regulatory cells at the clinical trials crossroad for the treatment of Autoimmune Disease; emphasis on type 1 diabetes therapy. Front Immunol 10:148
Chauhan A et al (2018) Interplay of vitamin D with T regulatory cells (FOXP3 + Treg) and thymic stromal lymphopoietin (TSLP) in children with atopic diseases. MOJ Immunol 6:95–98
Stockenhuber K et al (2018) Foxp3(+) T reg cells control psoriasiform inflammation by restraining an IFN-I-driven CD8(+) T cell response. J Exp Med 215(8):1987–1998
Saheb Sharif-Askari F et al (2023) Increased blood immune regulatory cells in severe COVID-19 with autoantibodies to type I interferons. Sci Rep 13(1):17344
Keller P et al (2021) 861 reprogramming regulatory T cells (Treg) using a MALT1 inhibitor for cancer therapy. BMJ Specialist Journals
Butcher MJ et al (2016) Atherosclerosis-driven Treg Plasticity results in formation of a dysfunctional subset of Plastic IFNγ + Th1/Tregs. Circ Res 119(11):1190–1203
Guo Z et al (2019) Inhibition of Cdk8/Cdk19 activity promotes Treg Cell differentiation and suppresses autoimmune diseases. Front Immunol 10:1988
MA X et al (2023) AB0564 TIGIT ACTIVATOR ALLEVIATES SYSTEMIC LUPUS ERYTHEMATOSUS BY NEGATIVELY REGULATING TH1-LIKE TREG CELL DIFFERENTIATION. BMJ Publishing Group Ltd
Hu M et al (2018) Low dose IL-2 and Rapamycin leads to prolongation of human islet allograft survival by inhibition of IFN-gamma + T cells and expansion of FOXP3 + CD25 + Tregs in a humanized islet transplant mouse model. Transplantation 102:S454
Vitale S et al (2021) Type I interferons induce peripheral T regulatory cell differentiation under tolerogenic conditions. Int Immunol 33(2):59–77
Scarsi M et al (2013) SAT0122 reduction of Peripheral Blood G-Ifn and IL-17 producing T cells after therapy with Abatacept for Rheumatoid Arthritis. Ann Rheum Dis 72(Suppl 3):A622–A622
Stazzoni S et al (2022) Novel poxin stable cGAMP-Derivatives are remarkable STING agonists. Angew Chem Int Ed 61(40):e202207175
Zaidi AH et al (2021) Intratumoral immunotherapy with STING agonist, ADU-S100, induces CD8 + T-cell mediated anti-tumor immunity in an esophageal adenocarcinoma model. Oncotarget 12(4):292–303
Ager CR et al (2017) Intratumoral STING activation with T-cell checkpoint modulation generates systemic antitumor immunity. Cancer Immunol Res 5(8):676–684
Falahat R et al (2019) STING signaling in melanoma cells shapes antigenicity and can promote antitumor T-cell activity. Cancer Immunol Res 7(11):1837–1848
Nakamura T et al (2021) STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation Journal for immunotherapy of cancer, 9(7)
Tian Z et al (2022) Cancer immunotherapy strategies that target the cGAS-STING pathway. Front Immunol 13:996663
Parkes EE et al (2022) Activation of a cGAS-STING-mediated immune response predicts response to neoadjuvant chemotherapy in early breast cancer. Br J Cancer 126(2):247–258
Amouzegar A et al (2021) STING agonists as cancer therapeutics. Cancers 13(11):2695
Conlon J et al (2013) Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol 190(10):5216–5225
Graham PT et al (2022) The STING agonist, DMXAA, reduces tumor vessels and enhances mesothelioma tumor antigen presentation yet blunts cytotoxic T cell function in a murine model. Front Immunol 13:969678
Del Prete A et al (2023) Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol Immunol 20(5):432–447
Graham PT et al (2022) The STING agonist, DMXAA, reduces tumor vessels and enhances mesothelioma tumor antigen presentation yet blunts cytotoxic T cell function in a murine model. Front Immunol, 13
Larkin B et al (2017) Cutting Edge: activation of STING in T cells induces type I IFN responses and cell death. J Immunol 199(2):397–402
Gan Y et al (2022) The cGAS/STING pathway: a novel target for cancer therapy. Front Immunol 12:795401
Tripathi S et al (2022) cGAS-STING pathway targeted therapies and their applications in the treatment of high-grade glioma. F1000Res 11:1010
Papaevangelou E et al (2023) Cyto-IL-15 synergizes with the STING agonist ADU-S100 to eliminate prostate tumors and confer durable immunity in mouse models. Front Immunol 14:1196829
Kong X et al (2023) STING as an emerging therapeutic target for drug discovery: perspectives from the global patent landscape. J Adv Res 44:119–133
Luo K et al (2022) Activation of stimulation of interferon genes (STING) signal and cancer immunotherapy. Molecules 27(14):4638
Kim DS et al (2021) E7766, a macrocycle-bridged stimulator of Interferon genes (STING) agonist with potent Pan‐genotypic activity. ChemMedChem 16(11):1741–1744
Kazerounian S et al (2022) BRG399, a small molecule modulator of UBE2K demonstrated dose-dependent anti-cancer efficacy in an in vivo model for gastric cancer. Cancer Res 82(12Supplement):5320–5320
Huang K-C et al (2019) Discovery and characterization of E7766, a novel macrocycle-bridged STING agonist with pan-genotypic and potent antitumor activity through intravesical and intratumoral administration. Cancer Res 79(13Supplement):3269–3269
Zhang Z et al (2022) Multifaceted functions of STING in human health and disease: from molecular mechanism to targeted strategy. Signal Transduct Target Ther 7(1):394
Du H, Xu T, Cui M (2021) cGAS-STING signaling in cancer immunity and immunotherapy, vol 133. Biomedicine & Pharmacotherapy, p 110972
Zhang R, Kang R, Tang D (2021) The STING1 network regulates autophagy and cell death. Signal Transduct Target Ther 6(1):208
Huang K-C et al (2020) Demonstration of E7766, a novel STING agonist, as a potent immunotherapy in BCG-insensitive non-muscle invasive bladder cancer models via intravesical administration. Cancer Res 80(16Supplement):592–592
Pan B-S et al (2020) An orally available non-nucleotide STING agonist with antitumor activity. Science 369(6506):eaba6098
Xuan C, Hu R (2023) Chemical Biology perspectives on STING agonists as Tumor Immunotherapy. ChemMedChem 18(23):e202300405
Lin H et al (2023) A non-nucleotide STING agonist MSA-2 synergized with manganese in enhancing STING activation to Elicit Potent Anti-RNA Virus Activity in the cells. Viruses 15(11):2138
Yi M et al (2022) Combination of oral STING agonist MSA-2 and anti-TGF-β/PD-L1 bispecific antibody YM101: a novel immune cocktail therapy for non-inflamed tumors. J Hematol Oncol 15(1):142
Liu J, Huang X, Ding J (2021) Identification of MSA-2: an oral antitumor non-nucleotide STING agonist. Signal Transduct Target Ther 6(1):18
McKeage MJ et al (2009) Phase II study of ASA404 (vadimezan, 5,6-dimethylxanthenone-4-acetic acid/DMXAA) 1800 mg/m(2) combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Lung Cancer 65(2):192–197
Pan ST et al (2015) Proteomic response to 5,6-dimethylxanthenone 4-acetic acid (DMXAA, vadimezan) in human non-small cell lung cancer A549 cells determined by the stable-isotope labeling by amino acids in cell culture (SILAC) approach. Drug Des Devel Ther 9:937–968
Huang S et al (2023) Disulfiram combined with chemoimmunotherapy potentiates pancreatic cancer treatment efficacy through the activation of cGAS-STING signaling pathway via suppressing PARP1 expression. Am J Cancer Res 13(5):2055
Huang S et al (2023) Disulfiram combined with chemoimmunotherapy potentiates pancreatic cancer treatment efficacy through the activation of cGAS-STING signaling pathway via suppressing PARP1 expression. Am J Cancer Res 13(5):2055–2065
Han Y, Liu D, Li L (2020) PD-1/PD-L1 pathway: current researches in cancer. Am J cancer Res 10(3):727
Luo K et al (2022) Activation of Stimulation of Interferon genes (STING) Signal and Cancer Immunotherapy. Molecules, 27(14)
Le Naour J et al (2020) Trial watch: STING agonists in cancer therapy. Oncoimmunology 9(1):1777624
Lee D et al (2022) Harnessing cGAS-STING pathway for Cancer Immunotherapy: from bench to Clinic. Adv Ther 5(10):2200040
Chang W et al (2022) Discovery of MK-1454: a potent cyclic dinucleotide stimulator of Interferon genes agonist for the treatment of Cancer. J Med Chem 65(7):5675–5689
Harrington K et al (2020) 972TiP phase II study of intratumoral MK-1454 plus pembrolizumab compared with pembrolizumab monotherapy as first-line treatment for metastatic or unresectable, recurrent head and neck squamous cell carcinoma. Ann Oncol 31:S683
Meric-Bernstam F et al (2019) Phase ib study of MIW815 (ADU-S100) in combination with spartalizumab (PDR001) in patients (pts) with advanced/metastatic solid tumors or lymphomas. American Society of Clinical Oncology
Liu C et al (2022) A phase II study of anlotinib combined with etoposide and platinum-based regimens in the first-line treatment of extensive-stage small cell lung cancer. Thorac Cancer 13(10):1463–1470
Jiang S et al (2021) Carboplatin versus cisplatin in combination with etoposide in the first-line treatment of small cell lung cancer: a pooled analysis. BMC Cancer 21(1):1–7
Zimmermann S et al (2018) Immune checkpoint inhibitors in the management of lung cancer. Am Soc Clin Oncol Educational Book 38:682–695
Ortega-Franco A et al (2021) First-line immune checkpoint inhibitors for extensive stage small-cell lung cancer: clinical developments and future directions. ESMO open 6(1):100003
Yano K, Shiotani B (2023) Emerging strategies for cancer therapy by ATR inhibitors. Cancer Sci 114(7):2709–2721
Venugopala KN (2022) Targeting the DNA damage response Machinery for Lung Cancer Treatment. Pharmaceuticals (Basel), 15(12)
Sen T (2023) STING pathway activation by ATR inhibition potentiates the antitumor immune response to anti-PD-L1 antibody in small cell lung cancer. Cancer Res 83(7Supplement):2265–2265
Motedayen Aval L et al (2020) Challenges and opportunities in the clinical development of STING agonists for cancer immunotherapy. J Clin Med 9(10):3323
Hu Q et al (2021) Cytosolic sensor STING in mucosal immunity: a master regulator of gut inflammation and carcinogenesis. J Experimental Clin Cancer Res 40:1–11
Weston AS et al (2023) Abstract LB323: inhibition of ENPP1 using small molecule, SR-8541A, enhances the effect of checkpoint inhibition in cancer models. Cancer Res 83(8Supplement):LB323–LB323
Li B et al (2023) Immune checkpoint inhibitors combined with targeted therapy: the recent advances and future potentials. Cancers 15(10):2858
Mohseni G et al (2021) The function of cGAS-STING pathway in treatment of pancreatic cancer. Front Immunol 12:781032
Tan YS et al (2018) Mitigating SOX2-potentiated immune escape of head and neck squamous cell carcinoma with a STING-inducing nanosatellite vaccine. Clin Cancer Res 24(17):4242–4255
Acknowledgements
This study was written by the authors.
Funding
No funding has been existed for this study.
Author information
Authors and Affiliations
Contributions
A.G. conceptualized the study. M.M. collected the data and wrote the manuscript. M.F. F.K. and G.B. wrote the text and revised it. A.G. edited and approved the text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
None to declare by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tabar, M.M.M., Fathi, M., Kazemi, F. et al. STING pathway as a cancer immunotherapy: Progress and challenges in activating anti-tumor immunity. Mol Biol Rep 51, 487 (2024). https://doi.org/10.1007/s11033-024-09418-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09418-4