Abstract
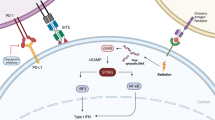
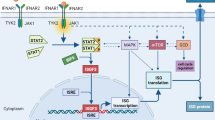
Acute myeloid leukemia (AML) is an aggressive hematologic cancer in adults. Some patients exhibit restricted T cell infiltration and do not respond to routine treatments. This may be prevented by enhancing adaptive immunity by stimulating innate immune cells inside the tumor microenvironment (TME). To activate the adaptive immunological reaction against tumors, type I interferons (IFNs) can promote the presentation of tumor-specific cytotoxic T lymphocyte (CTL) cell recruitment. During the activation of innate immunity, cyclic di-nucleotides (CDNs) bind to and stimulate the stimulator of interferon genes (STING), a protein localized inside the endoplasmic reticulum (ER) membrane, resulting in the expression of type I IFNs. The efficacy of STING agonists as effective stimulators of the anti-tumor response in AML is being investigated in numerous clinical studies. Therefore, the purpose of this investigation was to thoroughly review existing knowledge in this field and provide perspective into the clinical potential of STING agonists in AML.


Similar content being viewed by others
Availability of data and materials
It is not applicable.
References
Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–52.
Welch JS, Ley TJ, Link DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–78.
He X, Zhu Y, Yang L, et al. MgFe-LDH nanoparticles: a promising leukemia inhibitory factor replacement for self-renewal and pluripotency maintenance in cultured mouse embryonic stem cells. Adv Sci. 2021;8(9):2003535.
Perl AE. The role of targeted therapy in the management of patients with AML. Blood Adv. 2017;1(24):2281–94.
Stahl M, Goldberg AD. Immune checkpoint inhibitors in acute myeloid leukemia: novel combinations and therapeutic targets. Curr Oncol Rep. 2019;21(4):1–10.
Liu H, Gao Y, Vafaei S, Gu X, Zhong X. The prognostic value of plasma Cell-free DNA concentration in the prostate cancer: a systematic review and meta-analysis. Front Oncol. 2021;11:599602.
Liao D, Wang M, Liao Y, Li J, Niu T. A review of efficacy and safety of checkpoint inhibitor for the treatment of acute myeloid leukemia. Front Pharmacol. 2019;10:609.
Smits EL, Anguille S, Berneman ZN. Interferon α may be back on track to treat acute myeloid leukemia. Oncoimmunology. 2013;2(4):e23619.
Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–91.
Wu J, Sun L, Chen X, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–30.
Ashjari D, Karamali N, Rajabinejad M, et al. The axis of long non-coding RNA MALAT1/miR-1-3p/CXCR4 is dysregulated in patients with diabetic neuropathy. Heliyon. 2022;8(3):e09178.
Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol. 2015;15(12):760–70.
Azadeh H, Alizadeh-Navaei R, Rezaiemanesh A, Rajabinejad M. Immune-related adverse events (irAEs) in ankylosing spondylitis (AS) patients treated with interleukin (IL)-17 inhibitors: a systematic review and meta-analysis. Inflammopharmacology. 2022;30(2):435–51.
Zou M, Yang Z, Fan Y, et al. Gut microbiota on admission as predictive biomarker for acute necrotizing pancreatitis. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.988326.
Woo S-R, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33:445–74.
Su T, Zhang Y, Valerie K, et al. STING activation in cancer immunotherapy. Theranostics. 2019;9(25):7759.
Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87.
Sweet K, Asghari H. Acute myeloid leukemia: epidemiology and etiology. Acute Leukemias: Springer; 2021. p. 3–9.
Shafik NF, Ibraheem D, Selim MM, Allam RM, Fathalla LA. The prognostic significance of c-KIT mutations in core binding factor acute Myeloid Leukemia. Clin Lymphoma Myeloma Leuk. 2022;22(6):e363–75.
Juliusson G, Lehmann S, Lazarevic V. Epidemiology and etiology of AML. Acute Myeloid Leukemia: Springer; 2021. p. 1–22.
Guo Y, Wang W, Sun H. A systematic review and meta-analysis on the risk factors of acute myeloid leukemia. Transl Cancer Res. 2022;11(4):796.
Yi M, Li A, Zhou L, et al. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: estimates based on the global burden of disease study 2017. J Hematol Oncol. 2020;13(1):1–16.
Short NJ, Konopleva M, Kadia TM, et al. Advances in the treatment of acute myeloid leukemia: new drugs and new challengesadvances in AML therapeutics. Cancer Discov. 2020;10(4):506–25.
Magina KN, Pregartner G, Zebisch A, et al. Cytarabine dose in the consolidation treatment of AML: a systematic review and meta-analysis. Blood J Am Soc Hematol. 2017;130(7):946–8.
Othus M, Sekeres MA, Nand S, et al. Complete remissions (CRs) with Azacitidine regimens compared to Crs with 7+3 induction chemotherapy and the effect on overall survival. Blood. 2016;128(22):1613.
Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17(10):1142–9.
Weissmann C, Nagata S, Boll W, et al. Structure and expression of human IFN-α genes. Phil Trans R Soc Lond B Biol Sci. 1982;299(1094):7–28.
Hopfner K-P, Hornung V. Molecular mechanisms and cellular functions of cGAS–STING signalling. Nat Rev Mol Cell Biol. 2020;21(9):501–21.
Zhang X, Qu Y-Y, Liu L, et al. Homocysteine inhibits pro-insulin receptor cleavage and causes insulin resistance via protein cysteine-homocysteinylation. Cell Rep. 2021;37(2):109821.
Samimi Z, Kardideh B, Zafari P, et al. The impaired gene expression of adenosine monophosphate-activated kinase (AMPK), a key metabolic enzyme in leukocytes of newly diagnosed rheumatoid arthritis patients. Mol Biol Rep. 2019;46(6):6353–60.
Motwani M, Pesiridis S, Fitzgerald KA. DNA sensing by the cGAS–STING pathway in health and disease. Nat Rev Genet. 2019;20(11):657–74.
Zaver SA, Woodward JJ. Cyclic dinucleotides at the forefront of innate immunity. Curr Opin Cell Biol. 2020;63:49–56.
Rajabinejad M, Lotfi R, Roghani SA, et al. Difference in the Cytomegalovirus-related clinical laboratory findings between patients with bone marrow and kidney transplantation. Res Mol Med. 2021;9(3):197.
Xu S, Tao H, Cao W, et al. Ketogenic diets inhibit mitochondrial biogenesis and induce cardiac fibrosis. Signal Transduct Target Ther. 2021;6(1):1–13.
Galluzzi L, Vanpouille-Box C, Bakhoum SF, Demaria S. Snapshot: cGAS-STING signaling. Cell. 2018;173(1):276-e1.
Qu Y-Y, Zhao R, Zhang H-L, et al. Inactivation of the AMPK–GATA3–ECHS1 pathway induces fatty acid synthesis that promotes clear cell renal cell carcinoma growth. Can Res. 2020;80(2):319–33.
An X, Zhu Y, Zheng T, et al. An analysis of the expression and association with immune cell infiltration of the cGAS/STING pathway in pan-cancer. Mol Ther Nucl Acids. 2019;14:80–9.
Liu N, Pang X, Zhang H, Ji P. The cGAS-STING pathway in bacterial infection and bacterial immunity. Front Immunol. 2022;12:814709.
Heipertz EL, Harper J, Walker WE. STING and TRIF contribute to mouse sepsis, depending on severity of the disease model. Shock Inj Inflamm Sepsis Lab Clin Approach. 2017;47(5):621–31.
Li Y, Wilson HL, Kiss-Toth E. Regulating STING in health and disease. J Inflamm. 2017;14(1):1–21.
Zhang X, Bai XC, Chen ZJ. Structures and mechanisms in the cGAS-STING innate immunity pathway. Immunity. 2020;53(1):43–53.
Li Y, Yao C-F, Xu F-J, et al. APC/CCDH1 synchronizes ribose-5-phosphate levels and DNA synthesis to cell cycle progression. Nat Commun. 2019;10(1):1–16.
Larkin B, Ilyukha V, Sorokin M, et al. Cutting edge: activation of STING in T cells induces type I IFN Responses and cell death. J Immunol. 2017;199(2):397–402.
Gui X, Yang H, Li T, et al. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019;567(7747):262–6.
Cheng Z, Dai T, He X, et al. The interactions between cGAS-STING pathway and pathogens. Signal Transduct Target Ther. 2020;5(1):1–15.
Hemphill WO, Simpson SR, Liu M, et al. TREX1 as a novel immunotherapeutic target. Front Immunol. 2021;12:660184.
Zhang X, Liu L, Chen WC, et al. Gestational leucylation suppresses embryonic T-box transcription factor 5 signal and causes congenital heart disease. Adv Sci. 2022;9(15):2201034.
Ablasser A, Hemmerling I, Schmid-Burgk JL, et al. TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. J Immunol. 2014;192(12):5993–7.
Yan N. Immune diseases associated with TREX1 and STING dysfunction. J Interf Cytokine Res. 2017;37(5):198–206.
Wang D, Zhao R, Qu YY, et al. Colonic lysine homocysteinylation induced by high-fat diet suppresses DNA damage repair. Cell Rep. 2018;25(2):398–412.
Frémond M-L, Hadchouel A, Berteloot L, et al. Overview of STING-associated vasculopathy with onset in infancy (SAVI) among 21 patients. J Allerg Clin Immunol Pract. 2021;9(2):803–18.
Khorgami MR, Moradian M, Omidi N, Moghadam MYA. Management of cardiovascular disorders in patients with Noonan Syndrome: a case report. J Tehran Univ Heart Center. 2017;12(4):184.
Tabib A, Khorgami MR, Meraji M, Omidi N, Mirmesdagh Y. Accuracy of Doppler-derived indices in predicting pulmonary vascular resistance in children with pulmonary hypertension secondary to congenital heart disease with left-to-right shunting. Pediatr Cardiol. 2014;35(3):521–9.
Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–8.
Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–55.
Vafaei S, Saeednejad Zanjani L, Habibi Shams Z, et al. Low expression of Talin1 is associated with advanced pathological features in colorectal cancer patients. Sci Rep. 2020;10(1):1–18.
Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13(18):5256–61.
Iranshahi N, Assar S, Amiri SM, et al. Decreased gene expression of Epstein-Barr Virus-Induced Gene 3 (EBI-3) may contribute to the pathogenesis of rheumatoid arthritis. Immunol Invest. 2019;48(4):367–77.
Zafari P, Taghadosi M, Faramarzi F, Rajabinejad M, Rafiei A. Dimethyl fumarate inhibits fibroblast like synoviocytes-mediated inflammation and joint destruction in rheumatoid arthritis. Inflammation. 2022. https://doi.org/10.1007/s10753-022-01759-1.
Fuertes MB, Kacha AK, Kline J, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208(10):2005–16.
Diamond MS, Kinder M, Matsushita H, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003.
Dunn GP, Bruce AT, Sheehan KC, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6(7):722–9.
Woo SR, Fuertes MB, Corrales L, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–42.
Ohminami H, Yasukawa M, Fujita S. HLA class I-restricted lysis of leukemia cells by a CD8+ cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood J Am Soc Hematol. 2000;95(1):286–93.
Zhang L, Chen X, Liu X, et al. CD40 ligation reverses T cell tolerance in acute myeloid leukemia. J Clin Invest. 2013;123(5):1999–2010.
Curran E, Chen X, Corrales L, et al. STING Pathway Activation stimulates potent immunity against Acute Myeloid Leukemia. Cell Rep. 2016;15(11):2357–66.
Matikainen S, Sareneva T, Ronni T, et al. Interferon∙- activates multiple STAT proteins and upregulates proliferation-associated IL-2R∙, c-myc, and pim-1 genes in human T cells. Blood J Am Soc Hematol. 1999;93(6):1980–91.
Downey CM, Aghaei M, Schwendener RA, Jirik FR. DMXAA causes tumor site-specific vascular disruption in murine non-small cell lung cancer, and like the endogenous non-canonical cyclic dinucleotide STING agonist, 2’3’-cGAMP, induces M2 macrophage repolarization. PLoS ONE. 2014;9(6):e99988.
Conlon J, Burdette DL, Sharma S, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol. 2013;190(10):5216–25.
Che X, Du XX, Cai X, et al. Single mutations reshape the structural correlation network of the DMXAA-human STING complex. J Phys Chem B. 2017;121(9):2073–82.
Song C, Liu D, Liu S, et al. SHR1032, a novel STING agonist, stimulates anti-tumor immunity and directly induces AML apoptosis. Sci Rep. 2022;12(1):8579.
Meric-Bernstam F, Sweis RF, Hodi FS, et al. Phase I Dose-escalation trial of MIW815 (ADU-S100), an intratumoral STING agonist, in patients with advanced/metastatic solid tumors or lymphomas. Clin Cancer Res. 2022;28(4):677–88.
Lee JB, Khan DH, Hurren R, et al. Venetoclax enhances T cell-mediated antileukemic activity by increasing ROS production. Blood. 2021;138(3):234–45.
Levis M. Midostaurin approved for FLT3-mutated AML. Blood J Am Soc Hematol. 2017;129(26):3403–6.
Lai CT, Chi CW, Wu SH, et al. Midostaurin modulates tumor microenvironment and enhances efficacy of anti-PD-1 against colon cancer. Cancers (Basel). 2022;14(19):4847. https://doi.org/10.3390/cancers14194847.
Kogan AA, Topper MJ, Dellomo AJ, et al. Activating STING1-dependent immune signaling in TP53 mutant and wild-type acute myeloid leukemia. Proc Natl Acad Sci USA. 2022;119(27):e2123227119.
Stavrou S, Aguilera AN, Blouch K, Ross SR. DDX41 recognizes RNA/DNA retroviral reverse transcripts and is critical for in vivo control of murine leukemia virus infection. MBio. 2018. https://doi.org/10.1128/mBio.00923-18.
Singh RS, Vidhyasagar V, Yang S, et al. DDX41 is required for cGAS-STING activation against DNA virus infection. Cell Rep. 2022;39(8):110856.
Schieven G, Brown J, Swanson J, et al. editors 2018 Preclinical characterization of BMS-986301, a differentiated STING agonist with robust antitumor activity as monotherapy or in combination with anti-PD-1. Proceedings of the 33rd Annual Meeting & Pre-Conference Programs of the Society for Immunotherapy of Cancer (SITC 2018), Washington, DC, USA.
Challa SV, Zhou S, Sheri A, et al. Preclinical studies of SB 11285, a novel STING agonist for immuno-oncology. Am Soc Clin Oncol. 2017;35(15):e14616.
Endo A, Kim D-S, Huang K-C, et al. Discovery of E7766: A representative of a novel class of macrocycle-bridged STING agonists (MBSAs) with superior potency and pan-genotypic activity. Cancer Res. 2019;79(13):4456.
Adam M, Yu J, Plant R, et al. Sting agonist GSK3745417 induces apoptosis, antiproliferation, and cell death in a panel of human AML cell lines and patient samples. Blood. 2022;140(1):11829.
Funding
This study was supported by grants from Health Bureau Foundation of Zhejiang Province (2020KY015) and Education Foundation of Zhejiang Province (Y202146099).
Author information
Authors and Affiliations
Contributions
XS and FS contributed to the idea design and literature search. YP, QC, and XW wrote the manuscript. XL contributed to designing the figure.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
It is not applicable.
Informed consent
It is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, X., Peng, Y., Wang, X. et al. The stimulator of interferon genes (STING) agonists for treating acute myeloid leukemia (AML): current knowledge and future outlook. Clin Transl Oncol 25, 1545–1553 (2023). https://doi.org/10.1007/s12094-022-03065-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-03065-6