Abstract
Background
Pleurotus ostreatus, commonly known as the oyster mushroom, is a saprophytic fungus with many applications in biotechnology and medicine. This mushroom is a rich source of proteins, polysaccharides, and bioactive compounds that have been shown to possess anticancer, antioxidant, and immunomodulatory properties. In this study, we investigated the expression profile of laccase (POXA3) and β-glucan synthase (FKS) genes during different developmental stages in two strains of P. ostreatus.
Methods and results
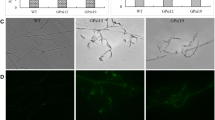
Cultural and morphological studies of the two strains were studied. DMR P115 strain recorded faster mycelial growth compared to the HUC strain. However, both strains produced white, thick fluffy mycelial growth with radiating margin. Morphological characteristics of the mushroom fruiting body were also higher in the DMR P115 strain. The expression of these genes was analyzed using quantitative real-time PCR (qPCR) and the results were compared to those of the reference gene β-actin. The expression of laccase (POXA3) was higher in the mycelial stage of DMR P115 and HUC strains indicating its role in the fruiting body development and substrate degradation. The expression of β-glucan synthase (FKS) was upregulated in the mycelium and mature fruiting body of the DMR P115 strain. In contrast, there was only significant upregulation in the mycelial stage of the HUC strain, which indicates its role in cell wall formation and the immunostimulatory properties of that strain.
Conclusion
The results deepen the understanding of the molecular mechanism of the fruiting body development in P. ostreatus and can be used as a foundation for future lines of research related to strain improvement of P. ostreatus.




Similar content being viewed by others
References
Sanchez C (2010) Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl Microbiol Biotechnol 85:1321–1337
Piska K, Sułkowska-Ziaja K, Muszyńska B (2017) Edible mushroom Pleurotus ostreatus (Oyster Mushroom) – its dietary significance and biological activity. Acta Sci Pol Hortorum Cultus 16(1): Article 1
Tsujiyama S, Ueno H (2013) Performance of wood-rotting fungi-based enzymes on enzymic saccharification of rice straw. J Sci Food Agric 93(11):2841–2848
Savoie JM, Salmones D, Mata G (2007) Hydrogen peroxide concentration measured in cultivation substrates during growth and fruiting of the mushrooms Agaricus bisporus and Pleurotus spp. J Sci Food Agric 87(7):1337–1344
Yildiz S, Umit C, Gezer E, Temiz A (2002) Some lignocellulosic wastes used as raw material in cultivation of the Pleurotus ostreatus culture mushroom. Process Biochem 38:301–306
Patel Y, Naraian R, Singh V (2012) Medicinal properties of Pleurotus species (oyster mushroom): a review. World J Fungal Plant Biol 3:1–12
Bellettini MB, Fiorda FA, Maieves HA, Teixeira GL, Avila S, Hornung PS, Junior AM, Ribani RH (2019) Factors affecting mushroom Pleurotus spp. Saudi J Biol Sci 26(4):633–646
Poniedziałek B, Mleczek M, Niedzielski P, Siwulski M, Gąsecka M, Kozak L, Komosa A, Rzymski P (2017) Bio-enriched Pleurotus mushrooms for deficiency control and improved antioxidative protection of human platelets? Eur Food Res Technol 243:2187–2198
Raman J, Jang KY, Oh YL, Oh M, Im JH, Lakshmanan H, Sabaratnam V (2021) Cultivation and nutritional value of prominent Pleurotus spp. Overv Mycobiology 49(1):1–14
Zhang L, Li CG, Liang HLM, Reddy N (2017) Bioactive mushroom polysaccharides: immunoceuticals to anticancer agents. J Nutraceuticals Food Sci 2(2):6
Kues U, Liu Y (2000) Fruiting body production in basidiomycetes. Appl Microbiol Biotechnol 54:141–152
Cohen R, Persky L, Hadar Y (2002) Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus. Appl Microbiol Biotechnol 58:582–594
Ohm RA, de Jong JF, Lugones LG, Aerts A, Kothe E et al (2010) Genome sequence of the model mushroom Schizophyllum commune. Nat Biotechnol 28:957–963
Sakamoto Y, Sato S, Ito M, Ando Y, Nakahori K, Muraguchi H (2018) Blue light exposure and nutrient conditions influence the expression of genes involved in simultaneous hyphal knot formation in Coprinopsis cinerea. Microbiol Res 217:81–90
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2009) Laccase: a neverending story. Cell Mol Life Sci 67(3):369–385
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30(2):215–242
Senthilvelan T, Kanagaraj J, Panda R (2016) Recent trends in fungal laccase for various industrial applications: an eco-friendly approach - A review. Biotechnol Bioprocess Eng 21:19–38
Fernandez-Fueyo E, Ruiz-Duenas FJ, Lopez-Lucendo MF, Perez-Boada M, Rencoret J, Gutierrez A, Pisabarro AG, Ramirez L, Martinez AT (2016) A secretomic view of woody and nonwoody lignocellulose degradation by Pleurotus ostreatus. Biotechnol Biofuels 9(1):49
Sakamoto Y, Nakade K, Yoshida K, Natsume S, Kazuhiro M, Sato S, van Peer A, Konno N (2015) Grouping of multicopper oxidases in Lentinula edodes by sequence similarities and expression patterns. AMB Express 5:63
Giardina P, Autore F, Faraco V, Festa G, Palmieri G, Piscitelli A, Sannia G (2007) Structural characterization of heterodimeric laccases from Pleurotus ostreatus. Appl Microbiol Biotechnol 75:1293–1300
Jiao X, Li G, Wang Y, Nie F, Cheng X, Abdullah M, Lin Y, Cai Y (2018) Systematic analysis of the Pleurotus ostreatus Laccase gene (PoLac) family and functional characterization of PoLac2 involved in the degradation of cotton-straw lignin. Molecules 23(4): Article 4
Rivera-Hoyos C, Morales-Alvarez E, Poutou-Pinales R, Pedroza-Rodriguez A, Rodriguez Vazquez R, Delgado-Boada J (2013) Fungal laccases. Fungal Biol Rev 27:67–82
Castanera R, Perez G, Omarini A, Alfaro M, Pisabarro A, Faraco V, Amore A, Ramirez L (2012) Transcriptional and enzymatic profiling of Pleurotus ostreatus laccase genes in submerged and solid-state fermentation cultures. Appl Environ Microbiol 78:4037–4045
Palmieri G, Cennamo G, Faraco V, Amoresano A, Sannia G, Giardina P (2003) Atypical laccase isoenzymes from copper supplemented Pleurotus ostreatus cultures. Enzyme Microb Technol 33(2–3):220–230
Reverberi M, Mario F, Tomati U (2005) β-Glucan synthase induction in mushrooms grown on olive mill wastewaters. Appl Microbiol Biotechnol 66(2):217–225
Robledo-Briones M, Ruiz-Herrera J (2012) Transcriptional regulation of the genes encoding chitin and β-1,3-glucan synthases from Ustilago maydis. Curr Microbiol 65(1):85–90
Cui FJ, Wu XH, Tao TL, Zan XY, Sun WJ, Mu DS, Yang Y, Wu D (2019) Functions of a glucan synthase gene GFGLS in mycelial growth and polysaccharide production of Grifola frondosa. J Agric Food Chem 67:8875–8883
Kaur R, Sharma M, Ji D, Xu M, Agyei D (2020) Structural features, modification, and functionalities of beta-glucan. Fibers 8:1
Fu Y, Dai Y, Yang C, Wei P, Song B, Yang Y, Sun L, Zhang ZW, Li Y (2017) Comparative transcriptome analysis identified candidate genes related to Bailinggu mushroom formation and genetic markers for genetic analyses and breeding. Sci Rep 7:9266
Rio DC, Ares M, Hannon GJ, Nilsen TW (2010) Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc 2010:prot5439
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Arora DS, Sharma RK (2010) Ligninolytic fungal laccases and their biotechnological applications. Appl Biochem Biotechnol 160(6):1760–1788
Rop O, Mlcek J, Jurikova T (2009) Beta-glucans in higher fungi and their health effects. Nutr Rev 67(11):624–631
Mishra RP, Shahid M, Pandey S, Pandey M, Deepshikha, Singh M (2015) Characterization of Pleurotus sp. of mushroom based on phenotypic, biochemical and yield parameter. Afr J Microbiol Res 9(13):934–937
Hoa H, Wang CL (2015) The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 43:14–23
Sardar H, Ali M, Ayyub C, Ahmad R (2015) Effects of different culture media, temperature and ph levels on the growth of wild and exotic Pleurotus species. Pak J Phytopathol 27(2):139–145
Shah ZA, Ashraf M, Ch MI (2004) Comparative study on cultivation and yield performance of oyster mushroom (Pleurotus ostreatus) on different substrates (wheat straw, leaves, saw dust). Pak J Nutr 3:158–160
Fan X, Zhou Y, Xiao Y, Xu Z, Bian Y (2014) Cloning, expression and phylogenetic analysis of a divergent laccase multigene family in Auricularia auricula-judae. Microbiol Res 169:453–462
Han ML, An Q, He SF, Zhang XL, Zhang MH, Gao XH et al (2020) Solid-state fermentation on poplar sawdust and corncob wastes for lignocellulolytic enzymes by different Pleurotus ostreatus strains. BioResources 15:4982–4995
Piscitelli A, Giardina P, Lettera V, Pezzella C, Sannia G, Faraco V (2011) Induction and transcriptional regulation of laccases in fungi. Curr Genomics 12(2):104–112
Ohga S, Royse DJ (2001) Transcriptional regulation of laccase and cellulase genes during growth and fruiting of Lentinula edodes on supplemented sawdust. FEMS Microbiol Lett 201:111–115
Chen J, Seviour R (2007) Medicinal importance of fungal β-(1→3), (1→6) glucans. Mycol Res 111(6):635–652
Synytsya A, Novak M (2014) Structural analysis of glucans. Ann Transl Med 2:17
Acknowledgements
We acknowledge the Kerala Agricultural University for providing all the facilities.
Funding
This study was funded by Kerala Agricultural University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
S., N.A., Thara, S.S., Soni, K.B. et al. Expression profiling of laccase and β-glucan synthase genes in Pleurotus ostreatus during different developmental stages. Mol Biol Rep 50, 7205–7213 (2023). https://doi.org/10.1007/s11033-023-08556-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08556-5