Abstract
Background
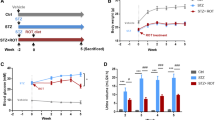
Reactive oxygen species (ROS) plays a vital role in the apoptosis of islet β-cells in type 2 diabetes mellitus (T2DM). Sirt3 (Sirtuin 3, a deacetylase) and FoxO1 (a transcription factor) might be involved in ROS production. This study was to investigate mechanism of ROS production and β-cell apoptosis in T2DM.
Methods
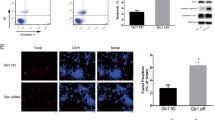
Oxidative stress and apoptosis in islets of db/db mice and high glucose cultured β-cells were observed by terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) assay and western blotting. Then, H2O2 was used to ascertain the effect of ROS on the expression of Sirt3. Meanwhile, FoxO1, antioxidant enzymes – catalase (CAT) and manganese superoxide dismutase (MnSOD) and β-cell apoptosis were also determined by western blotting. Finally, Sirt3 was knocked down to evaluate the effect on oxidative stress and apoptosis of β-cells.
Results
Under high glucose environment, enhanced ROS made a decrease of Sirt3 expression, which increased acetylation of FoxO1, thus reduced the expression of its target proteins –MnSOD and CAT, and further significantly increased ROS levels. Increased ROS finally led to the apoptosis of β-cells.
Conclusion
Down-regulation of Sirt3 plays an important role in the cyclic production of ROS and β-cell apoptosis. Targeting Sirt3 may be favorable for the treatment of T2DM.





Similar content being viewed by others
Abbreviations
- Catalase:
-
CAT
- Forkhead Box O1:
-
FoxO1
- hydrogen peroxide:
-
H2O2
- high glucose:
-
HG
- manganese superoxide dismutase:
-
MnSOD
- middle glucose:
-
MG
- normal glucose:
-
NG
- Reactive oxygen species:
-
ROS
- Sirtuin 3:
-
Sirt3
- type 2 diabetes mellitus:
-
T2DM
References
Tomita T (2016) Apoptosis in pancreatic beta-islet cells in Type 2 diabetes. Bosn J Basic Med Sci 16:162–179
Rehman K, Akash MSH (2017) Mechanism of generation of oxidative stress and pathophysiology of Type 2 diabetes mellitus: how are they interlinked? J Cell Biochem 118:3577–3585
Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA (2018) Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis 9:119
Green AS, Chen X, Macko AR, Anderson MJ, Kelly AC, Hart NJ, Lynch RM, Limesand SW (2012) Chronic pulsatile hyperglycemia reduces insulin secretion and increases accumulation of reactive oxygen species in fetal sheep islets. J Endocrinol 212:327–342
Rebelato E, Santos LR, Carpinelli AR, Rorsman P, Abdulkader F (2018) Short-term high glucose culture potentiates pancreatic beta cell function. Sci Rep 8:13061
Huang X, Shu H, Ren C, Zhu J (2022) SIRT3 improves bone regeneration and rescues diabetic fracture healing by regulating oxidative stress. Biochem Biophys Res Commun 604:109–115
Zhou C, Zhang Y, Jiao X, Wang G, Wang R, Wu Y (2021) SIRT3 alleviates neuropathic pain by deacetylating FoxO3a in the spinal dorsal horn of diabetic model rats. Reg Anesth Pain Med 46:49–56
Gong H, Liu J, Xue Z, Wang W, Li C, Xu F, Du Y, Lyu X (2022) SIRT3 attenuates coronary atherosclerosis in diabetic patients by regulating endothelial cell function. J Clin Lab Anal 36(8):e24586
Zhou Y, Chung ACK, Fan R, Lee HM, Xu G, Tomlinson B, Chan JCN, Kong APS (2017) Sirt3 deficiency increased the vulnerability of pancreatic beta cells to oxidative stress-induced dysfunction. Antioxid Redox Signal 27:962–976
Kim M, Lee JS, Oh JE, Nan J, Lee H, Jung HS, Chung SS, Park KS (2015) SIRT3 Overexpression attenuates Palmitate-induced pancreatic beta-cell dysfunction. PLoS ONE 10:e0124744
Benchoula K, Arya A, Parhar IS, Hwa WE (2021) FoxO1 signaling as a therapeutic target for type 2 diabetes and obesity. Eur J Pharmacol 891:173758
Qi R, Jiang R, Xiao H, Wang Z, He S, Wang L, Wang Y (2020) Ginsenoside Rg1 protects against d-galactose induced fatty liver disease in a mouse model via FOXO1 transcriptional factor. Life Sci 254:117776
Zhang X, Jiang L, Liu H (2021) Forkhead box protein O1: functional diversity and post-translational modification, a new therapeutic target? Drug Des Devel Ther 15:1851–1860
Chen J, Wang A, Chen Q (2017) SirT3 and p53 deacetylation in aging and cancer. J Cell Physiol 232:2308–2311
Zhang B, Cui S, Bai X, Zhuo L, Sun X, Hong Q, Fu B, Wang J, Chen X, Cai G (2013) SIRT3 overexpression antagonizes high glucose accelerated cellular senescence in human diploid fibroblasts via the SIRT3-FOXO1 signaling pathway. Age (Dordr) 35:2237–2253
Li J, Chen T, Xiao M, Li N, Wang S, Su H, Guo X, Liu H, Yan F, Yang Y, Zhang Y, Bu P (2016) Mouse Sirt3 promotes autophagy in AngII-induced myocardial hypertrophy through the deacetylation of FoxO1. Oncotarget 7:86648–86659
Wang JY, Nie YX, Dong BZ, Cai ZC, Zeng XK, Du L, Zhu X, Yin XX (2021) Quercetin protects islet beta-cells from oxidation-induced apoptosis via Sirt3 in T2DM. Iran J Basic Med Sci 24:629–635
Pena-Blanco A, Garcia-Saez AJ (2018) Bax, Bak and beyond - mitochondrial performance in apoptosis. FEBS J 285:416–431
Wang J, Yang X, Zhang J (2016) Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic beta cells. Cell Signal 28:1099–1104
Fridlyand LE, Philipson LH (2004) Does the glucose-dependent insulin secretion mechanism itself cause oxidative stress in pancreatic beta-cells? Diabetes 53:1942–1948
Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S (2007) Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 56:1783–1791
Li Z, Zhou Z, Huang G, Hu F, Xiang Y, He L (2013) Exendin-4 protects mitochondria from reactive oxygen species induced apoptosis in pancreatic beta cells. PLoS ONE 8:e76172
Rani S, Mehta JP, Barron N, Doolan P, Jeppesen PB, Clynes M, O’Driscoll L (2010) Decreasing Txnip mRNA and protein levels in pancreatic MIN6 cells reduces reactive oxygen species and restores glucose regulated insulin secretion. Cell Physiol Biochem 25:667–674
Hallows WC, Albaugh BN, Denu JM (2008) Where in the cell is SIRT3?–functional localization of an NAD+-dependent protein deacetylase. Biochem J 411:e11-13
Caton PW, Richardson SJ, Kieswich J, Bugliani M, Holland ML, Marchetti P, Morgan NG, Yaqoob MM, Holness MJ, Sugden MC (2013) Sirtuin 3 regulates mouse pancreatic beta cell function and is suppressed in pancreatic islets isolated from human type 2 diabetic patients. Diabetologia 56:1068–1077
Tang X, Li Y, Liu L, Guo R, Zhang P, Zhang Y, Zhang Y, Zhao J, Su J, Sun L, Liu Y (2020) Sirtuin 3 induces apoptosis and necroptosis by regulating mutant p53 expression in smallcell lung cancer. Oncol Rep 43:591–600
Wang Y, Chen J, Kong W, Zhu R, Liang K, Kan Q, Lou Y, Liu X (2017) Regulation of SIRT3/FOXO1 signaling pathway in rats with Non-alcoholic steatohepatitis by salvianolic acid B. Arch Med Res 48:506–512
Kitamura T (2013) The role of FOXO1 in beta-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol 9:615–623
Kim-Muller JY, Kim YJ, Fan J, Zhao S, Banks AS, Prentki M, Accili D (2016) FoxO1 deacetylation decreases fatty acid oxidation in beta-cells and sustains insulin secretion in diabetes. J Biol Chem 291:10162–10172
Mitchell S, Zhang P, Cannon M, Beaver L, Lehman A, Harrington B, Sampath D, Byrd JC, Lapalombella R (2021) Anti-tumor NAMPT inhibitor, KPT-9274, mediates gender-dependent murine anemia and nephrotoxicity by regulating SIRT3-mediated SOD deacetylation. J Hematol Oncol 14:101
Salvatori I, Valle C, Ferri A, Carri MT (2017) SIRT3 and mitochondrial metabolism in neurodegenerative diseases. Neurochem Int 109:184–192
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 81903681); the Xuzhou Science and Technology Program, China (Grant No. KC19029); the Director Project of Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy (Grant No. ZR-XY201502).
Author information
Authors and Affiliations
Contributions
CZ drafted the manuscript; LS and NY performed the in vitro experiments; DB participated the in vivo experiments; ZJ and XC performed immunofluorescence staining and imaging; LC provided some technical assistance; DL bought the agents; YX contributed to the interpretation of the data; WJ revised manuscript critically. All authors agreed with this submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethical approval
The animal study was reviewed and approved by Xuzhou Medical University on December 18, 2018 (approval number 201812W008).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, Z., Liu, S., Nie, Y. et al. Decreased Sirt3 contributes to cyclic production of reactive oxygen species and islet β-cell apoptosis in high glucose conditions. Mol Biol Rep 49, 10479–10488 (2022). https://doi.org/10.1007/s11033-022-07916-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07916-x