Abstract
Purpose
To investigate the role of thioredoxin 2 (Trx2) inhibition induced by intracellular methylglyoxal (MGO) in pancreatic beta-cell mitochondrial dysfunction and apoptosis.
Methods
Rat pancreatic beta-cell line INS-1 cells were treated with Glo1 siRNAs or exogenous MGO to increase intracellular MGO. AGEs formation was detected by ELISA and mitochondrial ROS was detected by probe MitoSOX. Transmission electron microscopy (TEM) analysis and ATP content were measured to evaluate mitochondrial function. Trx2 expression was manipulated by overexpression with recombinant Trx2 lentivirus or knockdown with Trx2 siRNAs, and effects on apoptosis and insulin secretion were measured by flow cytometry and ELISA, respectively.
Results
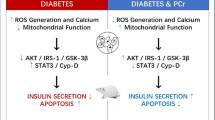
The increase of intracellular MGO by Glo1 blockage or MGO treatment led to advanced glycation end products (AGEs) overproduction, mitochondrial ROS increase, and insulin secretion paralysis. These were probably due to MGO-induced inhibition of mitochondrial Trx2. Trx2 inhibition by blockage of either Glo1 or Trx2 impaired mitochondrial integrity, inhibited cytochrome C oxidases subunit 1 and 4 (Cox1 and Cox4) expression and further reduced ATP generation, and all of these might lead to insulin paralysis; whereas Trx2 overexpression partially reversed MGO-induced oxidative stress, attenuated insulin secretion by preventing mitochondrial damage. Trx2 overexpression also retarded MGO-induced apoptosis of INS-1 cell through inhibiting ASK1 activation and downregulation of the ASK1-p38 MAPK pathway.
Conclusions
Our results reveal a possible mechanism for beta-cell oxidative damage upon intracellular MGO-induced Trx2 inactivation and mitochondrial dysfunction and apoptosis.






Similar content being viewed by others
References
M. Bensellam, D.R. Laybutt, J.C. Jonas, The molecular mechanisms of pancreatic β-cell glucotoxicity: recent findings and future research directions. Mol. Cell Endocrinol. (2012). https://doi.org/10.1016/j.mce.2012.08.003
J.C. Jonas, M. Bensellam, J. Duprez, H. Elouil, Y. Guiot, S.M.A. Pascal, Glucose regulation of islet stress responses and β-cell failure in type 2 diabetes. Diabetes Obes Metab. (2009). https://doi.org/10.1111/j.1463-1326.2009.01112.x
C.J. Rhodes, Type 2 diabetes—A matter of beta—cell life and death? Science 80 (2005). https://doi.org/10.1126/science.1104345
I. Allaman, M. Bélanger, P.J. Magistretti, Methylglyoxal, the dark side of glycolysis. Front. Neurosci. (2015). https://doi.org/10.3389/fnins.2015.00023
M.P. Kalapos, Where does plasma methylglyoxal originate from? Diabetes Res. Clin. Pract. (2013). https://doi.org/10.1016/j.diabres.2012.11.003
N. Lin, H. Zhang, Q. Su, Advanced glycation end-products induce injury to pancreatic beta cells through oxidative stress. Diabetes Metab. (2012). https://doi.org/10.1016/j.diabet.2012.01.003
C. Liu, X. Wan, T. Ye, F. Fang, X. Chen, Y. Chen, et al., Matrix metalloproteinase 2 contributes to pancreatic beta cell injury induced by oxidative stress. PLoS ONE 9 (2014). https://doi.org/10.1371/journal.pone.0110227
C. Liu, Y. Huang, Y. Zhang, X. Chen, X. Kong, Y. Dong, Intracellular methylglyoxal induces oxidative damage to pancreatic beta cell line INS-1 cell through Ire1α-JNK and mitochondrial apoptotic pathway. Free Radic. Res. 51 (2017). https://doi.org/10.1080/10715762.2017.1289376
P. Matafome, C. Sena, R. Seiça, Methylglyoxal, obesity, and diabetes. Endocrine (2013). https://doi.org/10.1007/s12020-012-9795-8
F. Giacco, X. Du, V.D. D’Agati, R. Milne, G. Sui, M. Geoffrion, et al., Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes (2014). https://doi.org/10.2337/db13-0316
J. Lu, A. Holmgren, The thioredoxin antioxidant system. Free Radic. Biol. Med. (2014). https://doi.org/10.1016/j.freeradbiomed.2013.07.036
T.F. Whayne, N. Parinandi, N. Maulik, Thioredoxins in cardiovascular disease. Can. J. Physiol. Pharmacol. (2015). https://doi.org/10.1139/cjpp-2015-0105
J.C. Lee, J.H. Park, I.H. Kim, G.S. Cho, J.H. Ahn, H.J. Tae, et al., Neuroprotection of ischemic preconditioning is mediated by thioredoxin 2 in the hippocampal CA1 region following a subsequent transient cerebral ischemia. Brain Pathol. (2017). https://doi.org/10.1111/bpa.12389
A.L. Dafre, J. Goldberg, T. Wang, D.A. Spiegel, P. Maher, Methylglyoxal, the foe and friend of glyoxalase and Trx/TrxR systems in HT22 nerve cells. Free Radic. Biol. Med. (2015). https://doi.org/10.1016/j.freeradbiomed.2015.07.005
J. Lu, E. Randell, Y.C. Han, K. Adeli, J. Krahn, Q.H. Meng, Increased plasma methylglyoxal level, inflammation, and vascular endothelial dysfunction in diabetic nephropathy. Clin. Biochem. (2011). https://doi.org/10.1016/j.clinbiochem.2010.11.004
X. Kong, M. zhe Ma, K. Huang, L. Qin, H. mei Zhang, Z. Yang, et al., Increased plasma levels of the methylglyoxal in patients with newly diagnosed type 2 diabetes. J. Diabetes (2014). https://doi.org/10.1111/1753-0407.12160
P.J. Beisswenger, Methylglyoxal in diabetes: link to treatment, glycaemic control and biomarkers of complications. Biochem. Soc. Trans. (2014). https://doi.org/10.1042/BST20130275
Z. Turk, Glycotoxines, carbonyl stress and relevance to diabetes and its complications. Physiol. Res. (2010)
M.J. Nokin, F. Durieux, P. Peixoto, B. Chiavarina, O. Peulen, A. Blomme, et al., Methylglyoxal, a glycolysis side-product, induces Hsp90 glycation and YAP- mediated tumor growth and metastasis. Elife (2016). https://doi.org/10.7554/eLife.19375
M. Yamamoto, E. Yamato, T. Shu-Ichi, F. Tashiro, H. Ikegami, J. Yodoi, et al., Transgenic expression of antioxidant protein thioredoxin in pancreatic β cells prevents progression of type 2 diabetes mellitus. Antioxid. Redox. Signal. (2008). https://doi.org/10.1089/ars.2007.1586
X.L. Wang, W.B. Lau, Y.X. Yuan, Y.J. Wang, W. Yi, T.A. Christopher, et al., Methylglyoxal increases cardiomyocyte ischemia-reperfusion injury via glycative inhibition of thioredoxin activity. Am. J. Physiol. Endocrinol. Metab. (2010). https://doi.org/10.1152/ajpendo.00215.2010
D.F.D. Mahmood, A. Abderrazak, K. El Hadri, T. Simmet, M. Rouis, The thioredoxin system as a therapeutic target in human health and disease. Antioxid. Redox. Signal. (2013). https://doi.org/10.1089/ars.2012.4757
H. Zhang, Y.M. Go, D.P. Jones, Mitochondrial thioredoxin-2/peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Arch. Biochem. Biophys. (2007). https://doi.org/10.1016/j.abb.2007.05.001
Q. Huang, H.J. Zhou, H. Zhang, Y. Huang, F. Hinojosa-Kirschenbaum, P. Fan, et al., Thioredoxin-2 inhibits mitochondrial reactive oxygen species generation and apoptosis stress kinase-1 activity to maintain cardiac function. Circulation (2015). https://doi.org/10.1161/CIRCULATIONAHA.114.012725
M. He, J. Cai, Y.M. Go, J.M. Johnson, W.D. Martin, J.M. Hansen, et al., Identification of thioredoxin-2 as a regulator of the mitochondrial permeability transition. Toxicol. Sci. (2008). https://doi.org/10.1093/toxsci/kfn116
K. Takeda, T. Noguchi, I. Naguro, H. Ichijo, Apoptosis Signal-Regulating Kinase 1 in Stress and Immune Response. Annu. Rev. Pharmacol. Toxicol. (2008). https://doi.org/10.1146/annurev.pharmtox.48.113006.094606
R. Zhang, R. Al-Lamki, L. Bai, J.W. Streb, J.M. Miano, J. Bradley, et al., Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ. Res. (2004). https://doi.org/10.1161/01.RES.0000130525.37646.a7
L. Yang, D. Wu, X. Wang, A.I. Cederbaum, Depletion of cytosolic or mitochondrial thioredoxin increases CYP2E1-induced oxidative stress via an ASK-1-JNK1 pathway in HepG2 cells. Free Radic. Biol. Med. (2011). https://doi.org/10.1016/j.freeradbiomed.2011.04.030
K.H. Huh, Y. Cho, B.S. Kim, J.H. Do, Y.J. Park, D.J. Joo, et al., The role of thioredoxin 1 in the mycophenolic acid-induced apoptosis of insulin-producing cells. Cell Death Dis. (2013). https://doi.org/10.1038/cddis.2013.247
Funding
This work was supported by the Grant of National Natural Science Foundation of China (No. 81800729) and Pudong New Area Health System Research Project (PW2018B-09).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, C., Cao, B., Zhang, Q. et al. Inhibition of thioredoxin 2 by intracellular methylglyoxal accumulation leads to mitochondrial dysfunction and apoptosis in INS-1 cells. Endocrine 68, 103–115 (2020). https://doi.org/10.1007/s12020-020-02191-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02191-x