Abstract
Background
Red chilli pepper (Capsicum annuum; RP) is a popular spice containing the active compound capsaicin. Indigenous gut bacteria and metabolism can affect host health. The functions of capsaicin, including the regulation of metabolic health and anti-oxidant properties, may be correlated with the gut microbiota.
Methods
To identify indigenous gut bacteria that are responsive to RP, Institute of Cancer Research mice fed a diet with no fibre or with 5% (w/w) RP for 14 days. Additionally, human stool samples collected from four healthy volunteers were incubated without (control) or with 2% (w/v) RP at 37 °C for 24 h. Microbiota in murine caecal samples and human faecal cultures were analysed using 16S rRNA (V4) amplicon sequencing.
Results
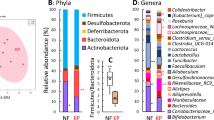
Compared with the microbiota in mice fed no-fibre diets, Lachnospiraceae spp.-, Muribaculaceae spp.-, and Phacaeicola vulgatus-like bacteria were defined as murine RP-responsive indigenous gut bacteria (RP-RIB). In the human faecal cultures, acetate and propionate levels were higher in RP cultures than in the control cultures. Subdoligranulum spp.-, Blautia spp.-, Faecalibacterium prausnitzii-, P. vulgatus-, and Prevotella copri-like bacteria were defined as human RP-RIB. Compared with control culture Fe-reducing power was increased in the culture with RP.
Conclusion
RP increases the amount of short-chain fatty acid–producing bacteria and beneficial gut bacteria in mouse and human faecal cultures. Overall, RP could have a positive effect on the host by altering the gut microbiota.







Similar content being viewed by others
References
Zhang D, Sun X, Battino M, Wei X, Shi J, Zhao L et al (2021) A comparative overview on chili pepper (capsicum genus) and sichuan pepper (zanthoxylum genus): from pungent spices to pharma-foods. Trends Food Sci Technol 117:148–162
Shi Z, Riley M, Brown A, Page A (2018) Chilli intake is inversely associated with hypertension among adults. Clin Nutr ESPEN 23:67–72
Duranova H, Valkova V, Gabriny L (2021) Chili peppers (Capsicum spp.): the spice not only for cuisine purposes: an update on current knowledge. Phytochem Rev. https://doi.org/10.1007/s11101-021-09789-7
Varghese S, Kubatka P, Rodrigo L, Gazdikova K, Caprnda M, Fedotova J et al (2017) Chili pepper as a body weight-loss food. Int J Food Sci Nutr 68:392–401
Chopan M, Littenberg B (2017) The association of hot red chili pepper consumption and mortality: a large population-based cohort study. PLoS ONE 12:e0169876
Fan Y, Pedersen O (2021) Gut microbiota in human metabolic health and disease. Gut microbiota in human metabolic health and disease. Nature Rev Microbiol 19:55–71
Ganal-Vonarburg SC, Hornef MW, Macpherson AJ (2020) Microbial–host molecular exchange and its functional consequences in early mammalian life. Science 368:604–607
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE et al (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563
Zaibi MS, Stocker CJ, O’Dowd J, Davies A, Bellahcene M, Cawthorne MA et al (2010) Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett 584:2381–2386
Geirnaert A, Calatayud M, Grootaert C, Laukens D, Devriese S, Smagghe G et al (2017) Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep 7:11450
Wu X, Xu N, Cheng C, McClements DJ, Chen X, Zou L, Liu W (2022) Encapsulation of hydrophobic capsaicin within the aqueous phase of water-in-oil high internal phase emulsions: controlled release, reduced irritation, and enhanced bioaccessibility. Food Hydrocoll 123:107184
Son J, Ren H, Gao Y, Lee C, Li S, Zhang F et al (2017) Dietary capsaicin improves glucose homeostasis and alters the gut microbiota in obese diabetic ob/ob mice. Front Physiol 8:602
Xia Y, Kuda T, Toyama A, Goto M, Fukunaga M, Takahashi H et al (2019) Detection and isolation of bacteria affected by dietary cumin, coriander, turmeric, and red chili pepper in the caecum of ICR mice. J Funct Foods 61:103467
Nagpal R, Wang S, Woods LC, Seshie O, Chung ST et al (2018) Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front Microbiol 9:2897
Faith JJ, McNulty NP, Rey FE, Gordon JI (2011) Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science 333:101–104
Xiang Q, Guo W, Tang X, Cui S, Zhang F, Liu X et al (2021) Capsaicin—the spicy ingredient of chili peppers: a review of the gastrointestinal effects and mechanisms. Trends Food Sci Technol 116:755–765
Xia Y, Kuda T, Nakamura S, Yamamoto M, Takahashi H, Kimura B (2022) Effects of soy protein and β-conglycinin on microbiota and in-vitro antioxidant and immunomodulatory capacities of human faecal cultures. Food Hydrocoll 127:107516
Takei MN, Kuda T, Taniguchi M, Nakamura S, Takahashi H, Kimura B (2020) Detection and isolation of low molecular weight alginate- and laminaran susceptible gut indigenous bacteria from ICR mice. Carbohydr Polym 238:116205
Lee G, Midorikawa Y, Kuda T, Harada M, Fujita S, Takahashi H et al (2022) In vitro antioxidant and anti-glycation properties of Sargassum horneri from golden tides on the South Korean coast and the effect on gut microbiota of mice fed a high-sucrose and low-fibre diet. J Appl Phycol 34:2211–2222
Shikano A, Kuda T, Shibayama J, Toyama A, Ishida Y, Takahashi H et al (2019) Effects of Lactobacillus plantarum Uruma-SU4 fermented green loofah on plasma lipid levels and gut microbiome of high-fat diet fed mice. Food Res Int 121:817–824
Sinclair L, Osman OA, Bertilsson S, Eiler A (2015) Microbial community composition and diversity via 16S rRNA gene amplicons: evaluating the Illumina platform. PLoS ONE 10:e0116955
Poncheewin W, Hermes GDA, van Dam JCJ, Koehorst JJ, Smidt H, Schaap PJ (2020) NG-Tax 2.0: a semantic framework for high-throughput amplicon analysis. Front Genet. https://doi.org/10.3389/fgene.2019.01366
Harada M, Kuda T, Nakamura S, Lee G, Takahashi H, Kimura B (2021) In vitro antioxidant and immunomodulation capacities of low-molecular weight-alginate- and laminaran-responsible gut indigenous bacteria. LWT Food Sci Technol 151:112127
Kim B, Shin J, Guevarra RB, Lee JH, Kim DW, Seol K et al (2017) Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol 27:2089–2093
O’Donoghue EM, Somerfield SD, Chen RKY, Tiffin HR, Hunter DA, Brummell DA (2020) Cell wall composition during expansion, ripening and postharvest water loss of red bell peppers (Capsicum annuum L.). Postharvest Biol Technol 168:111225
Careaga M, Fernández E, Dorantes L, Mota L, Jaramillo ME, Hernandez-Sanchez H (2003) Antibacterial activity of Capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. Int J Food Microbiol 83:331–335
Dorantes L, Colmenero R, Hernandez H, Mota L, Jaramillo ME, Fernandez E et al (2000) Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annum extracts. Int J Food Microbiol 57:125–128
Partula V, Mondot S, Torres MJ, Kesse-Guyot E, Deschasaux M, Assmann K (2019) Associations between usual diet and gut microbiota composition: results from the Milieu Intérieur cross-sectional study. Am J Clin Nutr 109:1472–1483
Hu Y, Peng J, Li F, Wong FS, Wen L (2018) Evaluation of different mucosal microbiota leads to gut microbiota-based prediction of type 1 diabetes in NOD mice. Sci Rep 8:1–13
Truax AD, Chen L, Tam JW, Cheng N, Guo H, Koblansky AA et al (2018) The inhibitory innate immune sensor NLRP12 maintains a threshold against obesity by regulating gut microbiota homeostasis. Cell Host Microbe 24:364–378
Zhang J, Song L, Wang Y, Liu C, Zhang L, Zhu S et al (2019) Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J Gastroenterol Hepatol 34:1368–1376
Haas KN, Blanchard JL (2017) Kineothrix alysoides, gen. nov., sp. nov., a saccharolytic butyrate-producer within the family Lachnospiraceae. Int J Syst Evol Microbiol 37:402–410
Hosny M, Abdallah RA, Khalil JB, Fontanini A, Baptiste E, Armstrong N et al (2019) Clostridium pacaense: a new species within the genus Clostridium. New Microb New Infect 28:6–10
Haas KN, Blanchard JL (2020) Reclassification of the Clostridium clostridioforme and Clostridium sphenoides clades as Enterocloster gen. nov. and Lacrimispora gen. nov., including reclassification of 15 taxa. Int J Syst Evol Microbiol 70:23–34
Tawtep S, Fukiya S, Lee J, Hagio M, Ogura Y, Hayashi T et al (2017) Isolation of six novel 7-oxo- or urso-type secondary bile acid-producing bacteria from rat cecal contents. J Biosci Bioeng 124:514–522
Takei M, Kuda T, Fukunaga M, Toyama A, Goto M, Takahashi H et al (2019) Effects of edible algae on caecal microbiomes of ICR mice fed a high-sucrose and low–dietary fibre diet. J Appl Phycol 31:3969–3978
Taniguchi M, Kuda T, Takei M, Takahashi H, Kimura B (2021) Effects of fermented Aphanizomenon flos-aquae on the caecal microbiome of mice fed a high-sucrose and low-dietary fibre diet. J Appl Phycol 33:397–407
Canfora EE, Meex RC, Venema K, Blaak EE (2019) Gut microbial metabolites in obesity, NAFLD and T2DM. Nature Rev Endocrinol 15:261–273
Abu-Ghazaleh N, Chia WJ, Gopalan V (2021) Intestinal microbiota and its association with colon cancer andred/processed meat consumption. J Gastroenterol Hepatol 36:75–88
Saito Y, Sato T, Nomoto K, Tsuji H (2018) Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiy125
Nakata T, Kyoui D, Takahashi H, Kimura B, Kuda T (2016) Inhibitory effects of laminaran and alginate on production ofputrefactive compounds from soy protein by intestinal microbiotain vitro and in rats. Carbohydr Polym 143:61–69
Hugenholtz F, de Vos WM (2018) Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci 75:149–160
Turner PV (2018) The role of the gut microbiota on animal model reproducibility. Animal Models Exp Med 1:109–115
Ren G, Xu L, Lu T, Zhang Y, Wang Y, Yin J (2019) Protective effects of lentinan on lipopolysaccharide induced inflammatory response in intestine of juvenile taimen (Hucho taimen, Pallas). Int J Biol Macromol 121:317–325
Venegas DP, De la Fuente MK, Landskron G, Gonzalez MJ, Quera R, Dijksta G et al (2019) Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 10:277
Kumari M, Singh P, Nataraj BH, Kokkiligadda A, Naithani H, Ali SA et al (2021) Fostering next-generation probiotics in human gut by targeted dietary modulation: an emerging perspective. Food Res Int 150:110716
Uchiyama J, Akiyama M, Hase K, Kumagai Y, Kim Y (2022) Gut microbiota reinforce host antioxidant capacity via the generation of reactive sulfur species. Cell Rep 38:110479
Trischler R, Roth J, Sorbara MT, Schlegel X, Müller V (2022) A functional Wood-Ljungdahl pathway devoid of a formate dehydrogenase in the gut acetogens Blautia wexlerae, Blautia luti and beyond. Environ Microbiol. https://doi.org/10.1111/1462-2920.16029
Oliver L, Ramió-Pujol S, Amoedo J, Malagón M, Sorrano M, Bahi A et al (2021) A novel grape-derived prebiotic selectively enhances abundance and metabolic activity of butyrate-producing bacteria in faecal samples. Front Microbiol 12:639948
Patcharatrakul T, Gonlachanvit S (2016) Chili peppers, curcumins, and prebiotics in gastrointestinal health and disease. Curr Gastroenterol Rep 18:1–11
Salyers AA, Vercellotti JR, West SEH, Wilkins TE (1977) Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol 33:319–322
Zmora N, Suez J, Elinav E (2019) You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol 16:35–56
Funding
This work was partially supported by the Yamazaki Spice Promotion Foundation, Tokyo, Japan.
Author information
Authors and Affiliations
Contributions
YX: Conceptualisation, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing—original draft, Visualisation. GL: Investigation, Data curation, MY: Investigation, HT: Conceptualisation, Methodology, Supervision. TK: Conceptualisation, Methodology, Validation, Formal analysis, Resources, Data curation, Writing—review & editing, Visualisation, Supervision, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This study was approved by the Animal Experiment Committee of the Tokyo University of Marine Science and Technology (Approval No. H31-5), and the Committee for Research Involving Human Subjects of the Tokyo University of Marine Science and Technology approved the study protocol (Approval no. R03-001).
Informed consent
All volunteers provided written informed consent before participating in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, Y., Lee, G., Yamamoto, M. et al. Detection of indigenous gut bacteria related to red chilli pepper (Capsicum annuum) in murine caecum and human faecal cultures. Mol Biol Rep 49, 10239–10250 (2022). https://doi.org/10.1007/s11033-022-07875-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07875-3