Abstract
Background
The frequency of triple-negative breast cancer (TNBC) incidence varies among different populations suggesting the involvement of genetic components towards TNBC development. Previous studies have reported that BRCA1/2 germline mutations confer a lifetime risk of developing TNBC. However, there is hardly any information regarding the common pathogenic variants (PVs) in BRCA1/2 genes that contribute to TNBC in the Indian population. Hence, we screened for PVs in BRCA1/2 and their association with clinico-pathological features in TNBC patients.
Methods and results
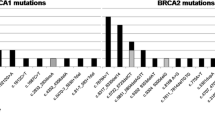
The study recruited 59 TNBC patients without hereditary breast and ovarian cancer (HBOC) from South India. The entire BRCA1 and BRCA2 genes were sequenced for the 59 patients using the Illumina HiSeq X Ten sequencer. Among the 59 TNBC genomic DNA samples sequenced, BRCA mutations were identified in 8 patients (13.6%), BRCA1 mutations in 6 patients, and BRCA2 mutations in 2 patients. Among the 6 BRCA1 mutations, three were c.68_69delAG (185delAG) mutation. Remarkably, all the TNBC patients with BRCA mutations exhibited higher-grade tumors (grade 2 or 3). However, among all the BRCA mutation carriers, only one patient with a BRCA2 mutation (p.Glu1879Lys) developed metastasis.
Conclusion
Our data advocates that South Indian women with higher grade TNBC tumors and without HBOC could be considered for BRCA mutation screening, thereby enabling enhanced decision-making and preventive therapy.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R et al (2018) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol 4:1553–1568
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer 109:1721–1728
Ray M, Polite BN (2010) Triple-negative breast cancers: a view from 10,000 feet. Cancer J 16:17–22
Jiagge E, Jibril AS, Chitale D, Bensenhaver JM, Awuah B, Hoenerhoff M et al (2016) Comparative analysis of breast cancer phenotypes in African American, White American, and west versus east African patients: correlation between African ancestry and triple-negative breast cancer. Ann Surg Oncol 23:3843–3849
Thakur KK, Bordoloi D, Kunnumakkara AB (2018) Alarming Burden of triple-negative breast cancer in India. Clin Breast Cancer 18:e393–e399
Chen H, Wu J, Zhang Z, Tang Y, Li X, Liu S et al (2018) Association between BRCA status and triple-negative breast cancer: a meta-analysis. Front Pharmacol 9:909
King MC, Marks JH, Mandell JB, New York Breast Cancer Study Group (2003) Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302:643–646
Stevens KN, Vachon CM, Couch FJ (2013) Genetic susceptibility to triple-negative breast cancer. Cancer Res 73:2025–2030
Daly MB, Axilbund JE, Buys S, Crawford B, Farrell CD et al (2010) Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Cancer Netw 8:562–594
Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Forero A, Giordano SH, Goldstein LJ (2011) Invasive breast cancer. J Natl Compr Cancer Netw 9(2):136–222
Comen E, Davids M, Kirchhoff T, Hudis C, Offit K, Robson M (2011) Relative contributions of BRCA1 and BRCA2 mutations to “triple-negative” breast cancer in Ashkenazi women. Breast Cancer Res Treat 129:185–190
Rosenthal E, Moyes K, Arnell C, Evans B, Wenstrup RJ (2015) Incidence of BRCA1 and BRCA2 non-founder mutations in patients of Ashkenazi Jewish ancestry. Breast Cancer Res Treat 149:223–227
Warner E, Foulkes W, Goodwin P, Meschino W, Blondal J, Paterson C et al (1999) Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst 91:1241–1247
Talhouet S, Peron J, Vuilleumier A, Friedlaender A, Viassolo V, Ayme A et al (2020) Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci Rep 10:7073
Levy-Lahad E, Catane R, Eisenberg S, Kaufman B, Hornreich G, Lishinsky E et al (1997) Founder BRCA1 and BRCA2 mutations in Ashkenazi Jews in Israel: frequency and differential penetrance in ovarian cancer and in breast-ovarian cancer families. Am J Hum Genet 60:1059–1067
Roa BB, Boyd AA, Volcik K, Richards CS (1996) Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet 14:185–187
Górski B, Jakubowska A, Huzarski T, Byrski T, Gronwald J, Grzybowska E et al (2004) A high proportion of founder BRCA1 mutations in polish breast cancer families. Int J Cancer 110:683–686
Krajc M, Teugels E, Zgajnar J, Goelen G, Besic N, Novakovic S et al (2008) Five recurrent BRCA1/2 mutations are responsible for cancer predisposition in the majority of Slovenian breast cancer families. BMC Med Genet 9:83
Johannesdottir G, Gudmundsson J, Bergthorsson JT, Arason A, Agnarsson BA, Eiriksdottir G et al (1996) High prevalence of the 999del5 mutation in Icelandic breast and ovarian cancer patients. Cancer Res 56:3663–3665
Rajkumar T, Soumittra N, Nancy NK, Swaminathan R, Sridevi V, Shanta V (2003) BRCA1, BRCA2 and CHEK2 (1100 del C) germline mutations in hereditary breast and ovarian cancer families in South India. Asian Pac J Cancer Prev 4:203–208
Valarmathi MT, Sawhney M, Deo SS, Shukla NK, Das SN (2004) Novel germline mutations in the BRCA1 and BRCA2 genes in Indian breast and breast-ovarian cancer families. Hum Mutat 23:205
Saxena S, Chakraborty A, Kaushal M, Kotwal S, Bhatanager D, Mohil RS et al (2006) Contribution of germline BRCA1 and BRCA2 sequence alterations to breast cancer in Northern India. BMC Med Genet 7:75
Vaidyanathan K, Lakhotia S, Ravishankar HM, Tabassum U, Mukherjee G, Somasundaram K (2009) BRCA1 and BRCA2 germline mutation analysis among Indian women from south India: identification of four novel mutations and high-frequency occurrence of 185delAG mutation. J Biosci 34:415–422
Juwle A, Saranath D (2012) BRCA1/BRCA2 gene mutations/SNPs and BRCA1 haplotypes in early-onset breast cancer patients of Indian ethnicity. Med Oncol 29:3272–3281
Kumar BV, Lakhotia S, Ankathil R, Madhavan J, Jayaprakash PG, Nair MK et al (2002) Germline BRCA1 mutation analysis in Indian breast/ovarian cancer families. Cancer Biol Ther 1:18–21
Hedau S, Jain N, Husain SA, Mandal AK, Ray G, Shahid M et al (2004) Novel germline mutations in breast cancer susceptibility genes BRCA1, BRCA2 and p53 gene in breast cancer patients from India. Breast Cancer Res Treat 88:177–186
Hansa J, Kannan R, Ghosh SK (2012) Screening of 185DelAG, 1014DelGT and 3889DelAG BRCA1 mutations in breast cancer patients from North-East India. Asian Pac J Cancer Prev 13:5871–5874
Mullineaux LG, Castellano TM, Shaw J, Axell L, Wood ME, Diab S et al (2003) Identification of germline 185delAG BRCA1 mutations in non-Jewish Americans of Spanish ancestry from the San Luis Valley, Colorado. Cancer 98:597–602
Laitman Y, Feng BJ, Zamir IM, Weitzel JN, Duncan P, Port D et al (2013) Haplotype analysis of the 185delAG BRCA1 mutation in ethnically diverse populations. Eur J Hum Genet 21:212–216
Drost R, Dhillon KK, van der Gulden H, van der Heijden I, Brandsma I, Cruz C et al (2016) BRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1. J Clin Invest 126:2903–2918
Gajalakshmi P, Natarajan TG, Selvi Rani D, Thangaraj K (2007) A novel BRCA1 mutation in an Indian family with hereditary breast/ovarian cancer. Breast Cancer Res Treat 101:3–6
Mehta A, Vasudevan S, Sharma SK, Kumar D, Panigrahi M, Suryavanshi M et al (2018) Germline BRCA1 and BRCA2 deleterious mutations and variants of unknown clinical significance associated with breast/ovarian cancer: a report from North India. Cancer Manag Res 10:6505–6516
Ahmad F, Natarajan S, Das BR (2020) Molecular spectrum of germline BRCA1 and BRCA2 mutation in a cohort of Indian cancer patients: a report from reference laboratory. J Mol Genet Med 14:467
Geredeli C, Yasar N, Sakin A (2019) Germline mutations in BRCA1 and BRCA2 in breast cancer patients with high genetic risk in Turkish population. Int J Breast Cancer 2019:9645147
Santonocito C, Rizza R, Paris I, Marchis L, Paolillo C, Tiberi G et al (2020) Spectrum of germline BRCA1 and BRCA2 variants identified in 2351 ovarian and breast cancer patients referring to a reference cancer hospital of Rome. Cancers 12:1286
Tihomirova L, Vaivade I, Fokina O, Peculis R, Mandrika I, Sinicka O et al (2014) BRCA1 gene-related hereditary susceptibility to breast and ovarian cancer in Latvia. Adv Med Sci 59:114–119
Lang GT, Shi JX, Hu X, Zhang CH, Shan L, Song CG et al (2017) The spectrum of BRCA mutations and characteristics of BRCA-associated breast cancers in China: screening of 2991 patients and 1,043 controls by next-generation sequencing. Int J Cancer 141:129–142
Thomassen M, Blanco A, Montagna M, Hansen TV, Pedersen IS, Gutiérrez-Enríquez S et al (2012) Characterization of BRCA1 and BRCA2 splicing variants: a collaborative report by ENIGMA consortium members. Breast Cancer Res Treat 132:1009–1023
Palmero EI, Carraro DM, Alemar B, Moreira M, Ribeiro-Dos-Santos Â, Abe-Sandes K et al (2018) The germline mutational landscape of BRCA1 and BRCA2 in Brazil. Sci Rep 8:9188
Pal T, Permuth-Wey J, Holtje T, Sutphen R (2004) BRCA1 and BRCA2 mutations in a study of African American breast cancer patients. Cancer Epidemiol Biomark Prev 13:1794–1799
Lindor NM, Guidugli L, Wang X, Vallée MP, Monteiro AN, Tavtigian S et al (2012) A review of a multifactorial probability-based model for classification of BRCA1 and BRCA2 variants of uncertain significance (VUS). Hum Mutat 33:8–21
Kadri M, Patel KM, Bhargava PA, Shah FD, Badgujar NV, Tarapara BV et al (2021) Mutational landscape for Indian hereditary breast and ovarian cancer cohort suggests need for identifying population specific genes and biomarkers for screening. Front Oncol 10:566
Seifert BA, O’Daniel JM, Amin K, Marchuk DS, Patel NM, Parker JS et al (2016) Germline analysis from tumor- germline sequencing dyads to identify clinically actionable secondary findings. Clin Cancer Res 22:4087–4094
Artioli G, Giannone G, Valabrega G, Maggiorotto F, Genta S, Pignata S et al (2021) Characteristics and outcome of BRCA mutated epithelial ovarian cancer patients in Italy: a retrospective multicenter study (MITO 21). Gynecol Oncol 161:755–761
Liu Y, Wang H, Wang X, Liu J, Li J, Wang X et al (2021) Prevalence and reclassification of BRCA1 and BRCA2 variants in a large, unselected Chinese Han breast cancer cohort. J Hematol Oncol 14:18
Cherbal F, Bakour R, Adane S, Boualga K, Benais-Pont G, Maillet P (2010) BRCA1 and BRCA2 germline mutations screening in Algerian breast/ovarian cancer families. Dis Markers 28:377–384
Jakimovska M, Maleva Kostovska I, Popovska-Jankovic K, Kubelka-Sabit K, Karadjozov M, Stojanovska L et al (2018) BRCA1 and BRCA2 germline variants in breast cancer patients from the Republic of Macedonia. Breast Cancer Res Treat 168:745–753
Heidemann S, Fischer C, Engel C, Fischer B, Harder L, Schlegelberger B et al (2012) Double heterozygosity for mutations in BRCA1 and BRCA2 in German breast cancer patients: implications on test strategies and clinical management. Breast Cancer Res Treat 134:1229–1239
Zuntini R, Ferrari S, Bonora E, Buscherini F, Bertonazzi B, Grippa M et al (2018) Dealing with BRCA1/2 unclassified variants in a cancer genetics clinic: does cosegregation analysis help? Front Genet 9:378
Thirthagiri E, Lee SY, Kang P, Lee DS, Toh GT, Selamat S et al (2008) Evaluation of BRCA1 and BRCA2 mutations and risk-prediction models in a typical Asian country (Malaysia) with a relatively low incidence of breast cancer. Breast Cancer Res 10:R59
Borg A, Haile RW, Malone KE, Capanu M, Diep A, Törngren T et al (2010) Characterization of BRCA1 and BRCA2 deleterious mutations and variants of unknown clinical significance in unilateral and bilateral breast cancer: the WECARE study. Hum Mutat 31:E1200–E1240
Rashid MU, Muhammad N, Bajwa S, Faisal S, Tahseen M, Bermejo JL et al (2016) High prevalence and predominance of BRCA1 germline mutations in Pakistani triple-negative breast cancer patients. BMC Cancer 16(1):673
Bhaskaran SP, Huang T, Rajendran BK, Guo M, Luo J, Qin Z et al (2021) Ethnic-specific BRCA1/2 variation within Asia population: evidence from over 78 000 cancer and 40 000 non-cancer cases of Indian, Chinese, Korean and Japanese populations. J Med Genet 58:752–759
Park HS, Park SJ, Kim JY, Kim S, Ryu J, Sohn J et al (2017) Next-generation sequencing of BRCA1/2 in breast cancer patients: potential effects on clinical decision-making using rapid, high-accuracy genetic results. Ann Surg Treat Res 92:331–339
Acknowledgements
The authors express their deep gratitude to all the study participants who volunteered in the study and the medical staff of Dr. G.V.N Cancer Institute. The authors also thank SASTRA—Deemed University for infrastructural support. The authors also thank the support of MEDGENOME, Bangalore, India during sample sequencing.
Funding
This study was funded by the Department of Science and Technology, SERB—Government of India (YSS/2015/001692) and Oncoclub, Chennai.
Author information
Authors and Affiliations
Contributions
TR: data curation, formal analysis, investigation, methodology, writing—original draft, writing—review & editing. AS: methodology, resources, supervision. AJ: data analysis, validation, software. KKR: methodology, resources. ST: investigation, supervision, review & editing; SV: review & editing; NRD: conceptualization, funding acquisition, project administration, resources, investigation, validation, supervision, writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Study proceeded with the approval of ethical committee Dr. G.V.N cancer Institute (GVNCI-IEC—ECR/436/INST/TN/2013).
Informed consent
Informed consent was obtained from all the individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajagopal, T., Seshachalam, A., Jothi, A. et al. Analysis of pathogenic variants in BRCA1 and BRCA2 genes using next-generation sequencing in women with triple negative breast cancer from South India. Mol Biol Rep 49, 3025–3032 (2022). https://doi.org/10.1007/s11033-022-07129-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07129-2