Abstract
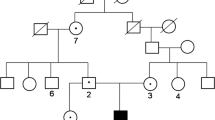
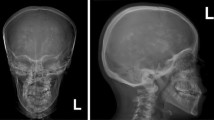
The carbonic anhydrase II (CAII) deficiency syndrome is a rare autosomal recessive osteopetrosis with renal tubular acidosis (RTA) and cerebral calcifications (MIM259730). CAII deficiency syndrome is caused by mutations in the gene CAII, which encodes the enzyme carbonic anhydrase II. CAII mutations are rarely reported in the Asian population. Here, we described two unrelated CAII deficiency families of Chinese Han origin with clinical and genetic analysis. Altogether, 106 subjects, including 2 probands, 4 unaffected family members from two non-consanguineous Chinese families, and 100 healthy controls were recruited. All seven exons and the exon-intron boundaries of the CAII gene were amplified and directly sequenced. Reverse transcription PCR (RT-PCR) was used to study the effect of splice site mutation. All clinical and biochemical parameters of the probands were collected. Two novel mutations of CAII gene were identified by mutational analysis: A nonsense mutation in exon 4 (c.T381C p.Y127X) in both families; a splice mutation at the splice donor site of intron 3 (c.350+2T>C, IVS3+2T>C) in one family. The splice-site mutation causes exon 3 skipping in patient’s mRNA resulting in an in-frame deletion and a novel premature stop codon. These mutations were predicted to result in a loss of function of CAII. This is the first report of CAII deficiency syndrome in Chinese population. Our findings extent the spectrum of CAII mutations observed in patients with CAII deficiency syndrome.





Similar content being viewed by others
References
Bolt RJ, Wennink JM, Verbeke JI, Shah GN, Sly WS, Bokenkamp A (2005) Carbonic anhydrase type ii deficiency. Am J Kidney Dis 46(A50):e71–e73
Chalhoub N, Benachenhou N, Rajapurohitam V, Pata M, Ferron M, Frattini A, Villa A, Vacher J (2003) Grey-lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat Med 9:399–406
Cleiren E, Benichou O, Van Hul E, Gram J, Bollerslev J, Singer FR, Beaverson K, Aledo A, Whyte MP, Yoneyama T, DeVernejoul MC, Van Hul W (2001) Albers-schonberg disease (autosomal dominant osteopetrosis, type ii) results from mutations in the clcn7 chloride channel gene. Hum Mol Genet 10:2861–2867
Del FA, Cappariello A, Teti A (2008a) Genetics, pathogenesis and complications of osteopetrosis. Bone 42:19–29
Del FA, Fornari R, Van Wesenbeeck L, de Freitas F, Timmermans JP, Peruzzi B, Cappariello A, Rucci N, Spera G, Helfrich MH, Van Hul W, Migliaccio S, Teti A (2008b) A new heterozygous mutation (r714c) of the osteopetrosis gene, pleckstrin homolog domain containing family m (with run domain) member 1 (plekhm1), impairs vesicular acidification and increases tracp secretion in osteoclasts. J Bone Miner Res 23:380–391
Eriksson AE, Jones TA, Liljas A (1988) Refined structure of human carbonic anhydrase ii at 2.0 a resolution. Proteins 4:274–282
Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson AK, Wallbrandt P, Zecca L, Notarangelo LD, Vezzoni P, Villa A (2000) Defects in tcirg1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet 25:343–346
Frattini A, Pangrazio A, Susani L, Sobacchi C, Mirolo M (2003) Chloride channel clcn7 mutations are responsible for severe recessive, dominant, and intermediate osteopetrosis. J Bone Miner Res 18:1740–1747
Hakansson K, Carlsson M, Svensson LA, Liljas A (1992) Structure of native and apo carbonic anhydrase ii and structure of some of its anion-ligand complexes. J Mol Biol 227:1192–1204
Halder P, Taraphder S (2013) Modeling the structure and proton transfer pathways of the mutant his-107-tyr of human carbonic anhydrase ii. J Mol Model 19:289–298
Hu PY, Roth DE, Skaggs LA, Venta PJ, Tashian RE, Guibaud P, Sly WS (1992) A splice junction mutation in intron 2 of the carbonic anhydrase ii gene of osteopetrosis patients from Arabic countries. Hum Mutat 1:288–292
Hu PY, Lim EJ, Ciccolella J, Strisciuglio P, Sly WS (1997) Seven novel mutations in carbonic anhydrase ii deficiency syndrome identified by sscp and direct sequencing analysis. Hum Mutat 9:383–387
Kornak U, Schulz A, Friedrich W, Uhlhaas S, Kremens B, Voit T, Hasan C, Bode U, Jentsch TJ, Kubisch C (2000) Mutations in the a3 subunit of the vacuolar h(+)-atpase cause infantile malignant osteopetrosis. Hum Mol Genet 9:2059–2063
Lange PF, Wartosch L, Jentsch TJ, Fuhrmann JC (2006) Clc-7 requires ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature 440:220–223
Nagai R, Kooh SW, Balfe JW, Fenton T, Halperin ML (1997) Renal tubular acidosis and osteopetrosis with carbonic anhydrase ii deficiency: pathogenesis of impaired acidification. Pediatr Nephrol 11:633–636
Nampoothiri S, Anikster Y (2009) Carbonic anhydrase ii deficiency a novel mutation. Indian Pediatr 46:532–534
Pangrazio A, Cassani B, Guerrini MM, Crockett JC, Marrella V (2012) Rank-dependent autosomal recessive osteopetrosis: characterization of five new cases with novel mutations. J Bone Miner Res 27:342–351
Pangrazio A, Fasth A, Sbardellati A, Orchard PJ, Kasow KA (2013) Snx10 mutations define a subgroup of human autosomal recessive osteopetrosis with variable clinical severity. J Bone Miner Res 28:1041–1049
Roth DE, Venta PJ, Tashian RE, Sly WS (1992) Molecular basis of human carbonic anhydrase ii deficiency. Proc Natl Acad Sci U S A 89:1804–1808
Shah GN, Bonapace G, Hu PY, Strisciuglio P, Sly WS (2004) Carbonic anhydrase ii deficiency syndrome (osteopetrosis with renal tubular acidosis and brain calcification): novel mutations in ca2 identified by direct sequencing expand the opportunity for genotype-phenotype correlation. Hum Mutat 24:272
Sly WS, Hewett-Emmett D, Whyte MP, Yu YS, Tashian RE (1983) Carbonic anhydrase ii deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A 80:2752–2756
Soda H, Yukizane S, Yoshida I, Aramaki S, Kato H (1995) Carbonic anhydrase ii deficiency in a Japanese patient produced by a nonsense mutation (tat–>tag) at tyr-40 in exon 2, (y40x). Hum Mutat 5:348–350
Stark Z, Savarirayan R (2009) Osteopetrosis. Orphanet J Rare Dis 4:5
Tashian RE (1989) The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays 10:186–192
Van Wesenbeeck L, Odgren PR, Coxon FP, Frattini A, Moens P, Perdu B, MacKay CA, Van Hul E, Timmermans JP, Vanhoenacker F, Jacobs R, Peruzzi B, Teti A, Helfrich MH, Rogers MJ, Villa A, Van Hul W (2007) Involvement of plekhm1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans. J Clin Invest 117:919–930
Whyte MP (1993) Carbonic anhydrase ii deficiency. Clin Orthop Relat Res 52–63
Acknowledgments
We appreciate our patients and their families for their participating in this study.
This study was supported by a grant from The Ministry of Science and Technology of the People’s Republic of China (National Science and Technology Major Projects for “Major New Drugs Innovation and Development” 2008ZX09312-016). National Natural Science Foundation of China (No. 81070687, 81470188 and 81170805), Beijing Natural Science Foundation (No. 7121012) and Scientific Research Foundation of Beijing Medical Development (No. 2007–3029). National Key Program of Clinical Science (WBYZ2011-873)
All listed authors have each made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; participated in drafting the manuscript or revising it critically for content; and have approved the final version of the submitted manuscript.
Dr. Qian-qian Pang and Prof. Wei-Bo Xia accept responsibility for the integrity of the data analysis.
Conflict of interest
All authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pang, Q., Qi, X., Jiang, Y. et al. Two novel CAII mutations causing carbonic anhydrase II deficiency syndrome in two unrelated Chinese families. Metab Brain Dis 30, 989–997 (2015). https://doi.org/10.1007/s11011-015-9660-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-015-9660-6