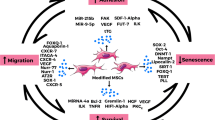
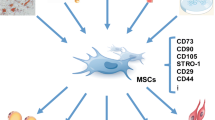
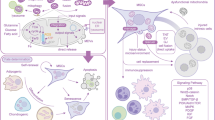
Abstract
Mesenchymal stem cells (MSCs) are considered to be a promising therapeutic material due to their capacities for self-renewal, multilineage differentiation, and immunomodulation and have attracted great attention in regenerative medicine. However, MSCs may lose their biological functions because of donor age or disease and environmental pressure before and after transplantation, which hinders the application of MSC-based therapy. As a major intracellular lysosome-dependent degradative process, autophagy plays a pivotal role in maintaining cellular homeostasis and withstanding environmental pressure and may become a potential therapeutic target for improving MSC functions. Recent studies have demonstrated that the regulation of autophagy is a promising approach for improving the biological properties of MSCs. More in-depth investigations about the role of autophagy in MSC biology are required to contribute to the clinical application of MSCs. In this review, we focus on the role of autophagy regulation by various physical and chemical factors on the biological functions of MSCs in vitro and in vivo, and provide some strategies for enhancing the therapeutic efficacy of MSCs.



Similar content being viewed by others
Abbreviations
- AD-MSCs:
-
Adipose tissue-derived mesenchymal stem cells
- ADM:
-
Adrenomedullin
- AMBRA1:
-
Activating molecule in Beclin-1 regulated autophagy
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- ATG:
-
Autophagy-related genes
- ATV:
-
Atorvastatin
- BECN1:
-
Bcl-2 interacting protein 1, also known as Beclin-1
- bFGF:
-
Basic fibroblast growth factor
- BMSCs:
-
Bone marrow mesenchymal stem cells
- CCL:
-
Chemokine (C-C motif) ligand
- CH:
-
Cholesterol
- CP-MSCs:
-
Placental chorionic plate-derived mesenchymal stem cells
- CXCL:
-
Chemokine (C-X-C motif) ligand
- CX3CL:
-
CX3C chemokine ligand
- Dex:
-
Dexamethasone
- FIP200:
-
FAK family-interacting protein of 200 kDa
- ENA-78:
-
Epithelial neutrophil-activating protein 78
- ER:
-
Endoplasmic reticulum
- GATA-4:
-
GATA-binding protein 4
- GDNF:
-
Glial cell line-derived neurotrophic factor
- GRO:
-
Growth-regulated oncogene
- HG:
-
High glucose
- HGF:
-
Hepatocyte growth factor
- HIF-1α:
-
Hypoxia-inducible factor-1α
- HLA:
-
Human leukocyte antigen
- HO-1:
-
Heme oxygenase-1
- H/SD:
-
Hypoxia/serum deprivation
- IDO:
-
Indoleamine 2,3-dioxigenase
- IGF-1:
-
Insulin-like growth factor-1
- IGF1R:
-
Insulin-like growth factor receptors
- IL:
-
Interleukins
- IL-1RA:
-
Interleukin-1 receptor antagonist
- IP3 :
-
Inositol 1,4,5-trisphosphate
- i-TAC:
-
Interferon-inducible T cell alpha chemoattractant
- LIF:
-
Leukemia inhibitory factor
- LIPUS:
-
Low-intensity pulsed ultrasound
- MAPK:
-
Mitogen-activated protein kinase
- MAP-LC3/LC3:
-
Microtubule-associated protein 1 light chain 3
- MCP:
-
Monocyte chemotactic protein
- MHC:
-
Major histocompatibility complex
- MIP:
-
Macrophage inflammatory protein
- MMP:
-
Metalloproteinase
- m-TORC1:
-
Mammalian target of rapamycin complex 1
- m-TORC2:
-
Mammalian target of rapamycin complex 2
- mTOR:
-
Mammalian target of rapamycin
- NGF:
-
Nerve growth factor
- NK cells:
-
Natural killer cells
- NO:
-
Nitric oxide
- Nrf2:
-
Nuclear factor erythoid-2-related factor 2
- OGD:
-
Oxygen-glucose deprivation
- PA:
-
Palmitate
- PDK1:
-
3-phosphoinositide-dependent protein kinase-1
- PDLSCs:
-
Periodontal ligament mesenchymal stem cells
- PE:
-
Phosphatidylethanolamine
- PGE2:
-
Prostaglandin E2
- PIGF:
-
Placental growth factor
- PI3P:
-
Phosphatidylinositol-3-phosphate
- PI3KC3–C1:
-
Class III phosphatidylinositol 3-kinase complex I
- PI3Ks:
-
Phosphatidylinositol 3-kinases
- PIP3 :
-
Phosphatidylinositol-3,4,5-trisphosphate
- PTEN:
-
Phosphatase and tensin homolog deleted on chromosome ten
- PTGS2:
-
Prostaglandin-endoperoxide synthase 2
- RANTES:
-
Regulated upon activation normal T cell expressed and secreted
- RAP:
-
Rapamycin
- RB1CC1:
-
RB1-inducible coiled-coil protein 1
- ROS:
-
Reactive oxygen species
- RSLT:
-
Reduced-size liver transplantation
- SCF:
-
Stem cell growth factor
- SDF-1:
-
Stromal-derived factor-1
- SLE:
-
Systemic lupus erythematosus
- SNARE:
-
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- SOD3:
-
Superoxide dismutase 3
- SOD:
-
Superoxide dismutase
- SSN:
-
Solid silica nanoparticles
- STC-1:
-
Stanniocalcin-1
- TCM:
-
Traditional Chinese medicine
- TIMP:
-
Tissue inhibitor of metalloproteinase
- TGF-β1:
-
Transforming growth factor-beta-1
- UCMSCs:
-
Umbilical cord-derived mesenchymal stem cells
- ULK1:
-
Unc51-like autophagy-activating kinase-1
- VAMP8:
-
Vesicle-associated membrane protein 8
- VCAM-1:
-
Vascular cell adhesion molecule-1
- VEGF:
-
Vascular endothelial growth factor
- VLA-4:
-
Very late antigen-4
- VPS34:
-
Vacuolar protein sorting protein 34
- VPS15:
-
Vacuolar protein sorting protein 15
References
Chen Y, Zeng Z, Sun J, Zeng H, Huang Y, Zhang Z (2015) Mesenchymal stem cell–conditioned medium prevents radiation-induced liver injury by inhibiting inflammation and protecting sinusoidal endothelial cells. J Radiat Res 56:700–708. https://doi.org/10.1093/jrr/rrv026
Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM (2017) Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cell Transl Med 6:2173–2185. https://doi.org/10.1002/sctm.17-0129
Fafianlabora J, Fernandezpernas P, Fuentes I, De Toro J, Oreiro N, Sangiaoalvarellos S, Mateos J, Arufe MC (2015) Influence of age on rat bone-marrow mesenchymal stem cells potential. Sci Rep 5:16765–16765. https://doi.org/10.1038/srep16765
Valentina T, Emanuela V, Claudia G (2016) Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int J Mol Sci 17:1164. https://doi.org/10.3390/ijms17071164
Hu C, Li L (2018) Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med 22:1428–1442. https://doi.org/10.1111/jcmm.13492
Janina F, Jagadeesh Kumar V, Ana RR, Gertrud S, Henning M, Magali C (2014) Determination of the chondrogenic differentiation processes in human bone marrow-derived mesenchymal stem cells genetically modified to overexpress transforming growth factor-β via recombinant adeno-associated viral vectors. Hum Gene Ther 25:1050–1060. https://doi.org/10.1089/hum.2014.091
Haque N, Kasim NHA, Rahman MT (2015) Optimization of pre-transplantation conditions to enhance the efficacy of mesenchymal stem cells. Int J Biol Sci 11:324–334. https://doi.org/10.7150/ijbs.10567
Jones KM, Saric N, Russell JP, Andoniadou CL, Scambler PJ, Basson MA (2015) CHD7 maintains neural stem cell quiescence and prevents premature stem cell depletion in the adult hippocampus. Stem Cells 33:196–210. https://doi.org/10.1002/stem.1822
Ferro F, Spelat R, Shaw G, Duffy N, Islam MN, O’Shea PM, O’Toole D, Howard L, Murphy JM (2019) Survival/adaptation of bone marrow-derived mesenchymal stem cells after long-term starvation through selective processes. Stem Cells 37:813–827. https://doi.org/10.1002/stem.2998
Sbrana FV, Cortini M, Avnet S, Perut F, Columbaro M, De Milito A, Baldini N (2016) The role of autophagy in the maintenance of stemness and differentiation of mesenchymal stem cells. Stem Cell Rev Rep 12:621–633. https://doi.org/10.1007/s12015-016-9690-4
Ma Y, Qi M, An Y, Zhang L, Yang R, Doro DH, Liu W, Jin Y (2018) Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell 17:669. https://doi.org/10.1111/acel.12709
Zheng Y, Hu CJ, Zhuo RH, Lei YS, Han NN, He L (2014) Inhibition of autophagy alleviates the senescent state of rat mesenchymal stem cells during long-term culture. Mol Med Rep 10:3003–3008. https://doi.org/10.3892/mmr.2014.2624
Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I (2007) Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 327:449–462. https://doi.org/10.1007/s00441-006-0308-z
Zhan XS, El-Ashram S, Luo DZ, Luo HN, Wang BY, Chen SF, Bai YS, Chen ZS, Liu CY, Ji HQ (2019) A comparative study of biological characteristics and transcriptome profiles of mesenchymal stem cells from different canine tissues. Int J Mol Sci 20:1485. https://doi.org/10.3390/ijms20061485
Liu Z, Lu S-J, Lu Y, Tan X, Zhang X, Yang M (2015) Transdifferentiation of human hair follicle mesenchymal stem cells into red blood cells by OCT4. Stem Cells Int 2015:389628. https://doi.org/10.1155/2015/389628
Sánchez L, Gutierrez-Aranda I, Ligero G, Rubio R, Muñoz-López M, García-Pérez JL, Ramos V, Real PJ, Bueno C, Rodríguez R (2015) Enrichment of human ESC-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem Cells 29:251–262. https://doi.org/10.1002/stem.569
Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, Selig M, Godwin J, Law K, Placidi C (2016) Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 183:993–1004. https://doi.org/10.4049/jimmunol.0900803
Centeno C, Markle J, Dodson E, Stemper I, Williams CJ, Hyzy M, Ichim T, Freeman M (2017) Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy. J Transl Med 15:197. https://doi.org/10.1186/s12967-017-1300-y
Nasr MB, Vergani A, Avruch J, Liu L, Kefaloyianni E, D’Addio F, Tezza S, Corradi D, Bassi R, Valderramavasquez A (2015) Co-transplantation of autologous MSCs delays islet allograft rejection and generates a local immunoprivileged site. Acta Diabetol 52:917–927. https://doi.org/10.1007/s00592-015-0735-y
Okolicsanyi RK, Camilleri ET, Oikari LE, Yu C, Cool SM, van Wijnen AJ, Griffiths LR, Haupt LM (2015) Human mesenchymal stem cells retain multilineage differentiation capacity including neural marker expression after extended in vitro expansion. PLoS One 10:e0137255. https://doi.org/10.1371/journal.pone.0137255
Gawronskakozak B, Manuel JA, Prpic V (2007) Ear mesenchymal stem cells (EMSC) can differentiate into spontaneously contracting muscle cells. J Cell Biochem 102:122–135. https://doi.org/10.1002/jcb.21286
Liu QX, Cheng GG, Wang ZW, Zhan SJ, Xiong BB, Zhao XM (2015) Bone marrow-derived mesenchymal stem cells differentiate into nerve-like cells in vitro after transfection with brain-derived neurotrophic factor gene. In Vitro Cell Dev Biol Anim 51:319–327. https://doi.org/10.1007/s11626-015-9875-1
Wu M, Zhou T, Liu H (2015) Ca2+ and EGF induce the differentiation of human embryo mesenchymal stem cells into epithelial-like cells. Cell Biol Int 39:852–857. https://doi.org/10.1002/cbin.10398
Hu C, Li L (2015) In vitro culture of isolated primary hepatocytes and stem cell-derived hepatocyte-like cells for liver regeneration. Protein Cell 6:562–574. https://doi.org/10.1007/s13238-015-0180-2
Liu S, Yuan M, Hou K, Zhang L, Zheng X, Zhao B, Sui X, Xu W, Lu S, Guo Q (2012) Immune characterization of mesenchymal stem cells in human umbilical cord Wharton’s jelly and derived cartilage cells. Cell Immunol 278:35–44. https://doi.org/10.1016/j.cellimm.2012.06.010
Bruno S, Deregibus MC, Camussi G (2015) The secretome of mesenchymal stromal cells: role of extracellular vesicles in immunomodulation. Immunol Lett 168:154–158. https://doi.org/10.1016/j.imlet.2015.06.007
Li N, Hua J (2017) Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci 74:2345–2360. https://doi.org/10.1007/s00018-017-2473-5
Wang Y, Chen X, Cao W, Shi Y (2014) Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol 15:1009. https://doi.org/10.1038/ni.3002
Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC (2016) Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol Biol 1416:123–146. https://doi.org/10.1007/978-1-4939-3584-0_7
Bassi EJ, de Almeida DC, Moraes-Vieira PM, Camara NO (2012) Exploring the role of soluble factors associated with immune regulatory properties of mesenchymal stem cells. Stem Cell Rev Rep 8:329–342. https://doi.org/10.1007/s12015-011-9311-1
Montemurro T, Vigano M, Ragni E, Barilani M, Parazzi V, Boldrin V, Lavazza C, Montelatici E, Banfi F, Lauri E, Giovanelli S, Baccarin M, Guerneri S, Giordano R, Lazzari L (2016) Angiogenic and anti-inflammatory properties of mesenchymal stem cells from cord blood: soluble factors and extracellular vesicles for cell regeneration. Eur J Cell Biol 95:228–238. https://doi.org/10.1016/j.ejcb.2016.04.003
Li Z, Wang J, Gao F, Zhang J, Tian H, Shi X, Lian C, Sun Y, Li W, Xu JY, Li P, Zhang J, Gao Z, Xu J, Wang F, Lu L, Xu GT (2016) Human adipose-derived stem cells delay retinal degeneration in Royal College of Surgeons rats through anti-apoptotic and VEGF-mediated neuroprotective effects. Curr Mol Med 16:553–566. https://doi.org/10.2174/1566524016666160607090538
Ohkouchi S, Block GJ, Katsha AM, Kanehira M, Ebina M, Kikuchi T, Saijo Y, Nukiwa T, Prockop DJ (2012) Mesenchymal stromal cells protect cancer cells from ROS-induced apoptosis and enhance the Warburg effect by secreting STC1. Mol Ther 20:417–423. https://doi.org/10.1038/mt.2011.259
Ishii M, Takahashi M, Murakami J, Yanagisawa T, Nishimura M (2019) Vascular endothelial growth factor-C promotes human mesenchymal stem cell migration via an ERK-and FAK-dependent mechanism. Mol Cell Biochem 455:185–193. https://doi.org/10.1007/s11010-018-3481-y
Li L, Zhang S, Zhang Y, Yu B, Xu Y, Guan Z (2009) Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol Biol Rep 36:725–731. https://doi.org/10.1007/s11033-008-9235-2
Jeong GJ, Song SY, Kang M, Go S, Sohn HS, Kim BS (2018) An injectable decellularized matrix that improves mesenchymal stem cell engraftment for therapeutic angiogenesis. ACS Biomater Sci Eng 4:2571–2581. https://doi.org/10.1021/acsbiomaterials.8b00617
Gonzalezking H, Garcia NA, Ontoriaoviedo I, Ciria M, Montero JA, Sepulveda P (2017) Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells 35:1747–1759. https://doi.org/10.1002/stem.2618
Kwon HM, Hur S-M, Park K-Y, Kim C-K, Kim Y-M (2014) Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vasc Pharmacol 63:19–28. https://doi.org/10.1016/j.vph.2014.06.004
Ali A, Akhter MA, Haneef K, Khan I, Naeem N, Habib R, Kabir N, Salim A (2015) Dinitrophenol modulates gene expression levels of angiogenic, cell survival and cardiomyogenic factors in bone marrow derived mesenchymal stem cells. Gene 555:448–457. https://doi.org/10.1016/j.gene.2014.10.045
Bandara N, Kong A, Sivakumaran P, Mitchel G, Wang LX, Lim S, Strappe P (2017) Nitric oxide directed reprogramming of rat bone marrow derived mesenchymal stem cells into endothelial-like cells via activation of WNT/β-catenin signaling. Mech Develop 145:S134–S135. https://doi.org/10.1016/j.mod.2017.04.374
Hynes B, Kumar AHS, Osullivan JF, Buneker CK, Leblond A, Weiss S, Schmeckpeper J, Martin K, Caplice NM (2013) Potent endothelial progenitor cell-conditioned media-related anti-apoptotic, cardiotrophic, and pro-angiogenic effects post-myocardial infarction are mediated by insulin-like growth factor-1. Eur Heart J 34:782–789. https://doi.org/10.1093/eurheartj/ehr435
Rabbani S, Soleimani M, Sahebjam M, Imani M, Haeri A, Ghiaseddin A, Nassiri SM, Majd Ardakani J, Tajik Rostami M, Jalali A, Ahmadi Tafti SH (2018) Simultaneous delivery of Wharton’s Jelly mesenchymal stem cells and insulin-like growth Factor-1 in acute myocardial infarction. Iran J. Pharm Res 17:426–441. https://doi.org/10.22037/IJPR.2018.2203
He JG, Li HR, Li BB, Xie QL, Yan D, Wang XJ (2019) Bone marrow mesenchymal stem cells overexpressing GATA-4 improve cardiac function following myocardial infarction. Perfusion 34:696–704. https://doi.org/10.1177/0267659119847442
Meirelles LD, Caplan AI, Nardi NB (2008) In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26:2287–2299. https://doi.org/10.1634/stemcells.2007-1122
Steingen C, Brenig F, Baumgartner L, Schmidt J, Schmidt A, Bloch W (2008) Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. Proc Natl Acad Sci U S A 44:1072–1084. https://doi.org/10.1016/j.yjmcc.2008.03.010
Pourjafar M, Saidijam M, Etemadi K, Najafi R (2017) All-trans retinoic acid enhances in vitro mesenchymal stem cells migration by targeting matrix metalloproteinases 2 and 9. Biotechnol Lett 39:1263–1268. https://doi.org/10.1007/s10529-017-2350-1
Vogel S, Peters C, Etminan N, Borger V, Schimanski A, Sabel M, Sorg RV (2013) Migration of mesenchymal stem cells towards glioblastoma cells depends on hepatocyte-growth factor and is enhanced by aminolaevulinic acid-mediated photodynamic treatment. Biochem Biophys Res Commun 431:428–432. https://doi.org/10.1016/j.bbrc.2012.12.153
Choi CH, Chung JY, Chung EJ, Sears JD, Lee JW, Bae DS, Hewitt SM (2016) Prognostic significance of annexin A2 and annexin A4 expression in patients with cervical cancer. BMC Cancer 16:448. https://doi.org/10.1186/s12885-016-2459-y
Oh M, Kim J, Kang C (2016) Protective effect of S-adenosyl-L-methionine on oxidative stress-induced apoptosis regulates Nrf2 via IGF-I in rat bone marrow mesenchymal stem cells. J Stem Cell Res Ther 6:1–9. https://doi.org/10.4172/2157-7633.1000323
Jang YH, You DH, Nam MJ (2015) Protective effects of HGF gene-expressing human mesenchymal stem cells in acetaminophen-treated hepatocytes. Growth Factors 33:319–325. https://doi.org/10.3109/08977194.2015.1080695
Xu G, Zhang Y, Zhang L, Ren G, Shi Y (2007) The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem Biophys Res Commun 361:745–750. https://doi.org/10.1016/j.bbrc.2007.07.052
Francois S, Mouiseddine M, Allenet-Lepage B, Voswinkel J, Douay L, Benderitter M, Chapel A (2013) Human mesenchymal stem cells provide protection against radiation-induced liver injury by antioxidative process, vasculature protection, hepatocyte differentiation, and trophic effects. Biomed Res Int 2013:151679. https://doi.org/10.1155/2013/151679
Ono M, Ohkouchi S, Kanehira M, Tode N, Kobayashi M, Ebina M, Nukiwa T, Irokawa T, Ogawa H, Akaike T (2015) Mesenchymal stem cells correct inappropriate epithelial–mesenchyme relation in pulmonary fibrosis using stanniocalcin-1. Mol Ther 23:549–560. https://doi.org/10.1038/mt.2014.217
Dong LH, Jiang YY, Liu YJ, Cui S, Xia CC, Qu C, Jiang X, Qu YQ, Chang PY, Liu F (2015) The anti-fibrotic effects of mesenchymal stem cells on irradiated lungs via stimulating endogenous secretion of HGF and PGE2. Sci Rep 5:8713. https://doi.org/10.1038/srep08713
Wang X, Wang H, Lu J, Feng Z, Liu Z, Song H, Wang H, Zhou Y, Xu J (2020) Erythropoietin-modified mesenchymal stem cells enhance anti-fibrosis efficacy in mouse liver fibrosis model. Tissue Eng Regen Med 3:1–11. https://doi.org/10.1007/s13770-020-00276-2
Jang YJ, An SY, Kim JH (2017) Identification of MFGE8 in mesenchymal stem cell secretome as an anti-fibrotic factor in liver fibrosis. BMB Rep 50:58–59. https://doi.org/10.5483/BMBRep.2017.50.2.012
An SY, Jang YJ, Lim HJ, Han J, Lee J, Lee G, Park J, Park SY, Kim JH, Do BR (2017) Milk fat globule-EGF factor 8, secreted by mesenchymal stem cells, protects against liver fibrosis in mice. Gastroenterology 152:1174–1186. https://doi.org/10.1053/j.gastro.2016.12.003
Wu Y, Peng Y, Gao D, Feng C, Yuan X, Li H, Wang Y, Yang L, Huang S, Fu X (2015) Mesenchymal stem cells suppress fibroblast proliferation and reduce skin fibrosis through a TGF-β3-dependent activation. Int J Low Extrem Wounds 14:50–62. https://doi.org/10.1177/1534734614568373
Ma Z, Song G, Zhao D, Liu D, Liu X, Dai Y, He Z, Qian D, Gong J, Meng H, Zhou BO, Yang T, Song Z (2019) Bone marrow-derived mesenchymal stromal cells ameliorate severe acute pancreatitis in rats via hemeoxygenase-1-mediated anti-oxidant and anti-inflammatory effects. Cytotherapy 21:162–174. https://doi.org/10.1016/j.jcyt.2018.11.013
Whone AL, Kemp K, Sun M, Wilkins A, Scolding NJ (2012) Human bone marrow mesenchymal stem cells protect catecholaminergic and serotonergic neuronal perikarya and transporter function from oxidative stress by the secretion of glial-derived neurotrophic factor. Brain Res 1431:86–96. https://doi.org/10.1016/j.brainres.2011.10.038
Morel E, Mehrpour M, Botti J, Dupont N, Hamai A, Nascimbeni AC, Codogno P (2017) Autophagy: a druggable process. Annu Rev Pharmacol Toxicol 57:375–398. https://doi.org/10.1146/annurev-pharmtox-010716-104936
Sridhar S, Botbol Y, Macian F, Cuervo AM (2012) Autophagy and disease: always two sides to a problem. J Pathol 226:255–273. https://doi.org/10.1002/path.3025
Yu L, Chen Y, Tooze SA (2018) Autophagy pathway: cellular and molecular mechanisms. Autophagy 14:207–215. https://doi.org/10.1080/15548627.2017.1378838
Hurley JH, Young LN (2017) Mechanisms of autophagy initiation. Annu Rev Biochem 86:225–244. https://doi.org/10.1146/annurev-biochem-061516-044820
Backer JM (2016) The intricate regulation and complex functions of the class III phosphoinositide 3-kinase Vps34. Biochem J 473:2251–2271. https://doi.org/10.1042/BCJ20160170
Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S (2010) Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90:1383–1435. https://doi.org/10.1152/physrev.00030.2009
Jhanwar-Uniyal M, Jeevan D, Neil J, Shannon C, Albert L, Murali R (2013) Deconstructing mTOR complexes in regulation of Glioblastoma Multiforme and its stem cells. Adv Biol Regul 53:202–210. https://doi.org/10.1016/j.jbior.2012.10.001
Bento CF, Renna M, Ghislat G, Puri C, Ashkenazi A, Vicinanza M, Menzies FM, Rubinsztein DC (2016) Mammalian autophagy: how does it work? Annu Rev Biochem 85:685–713. https://doi.org/10.1146/annurev-biochem-060815-014556
Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK (2014) Autophagy and apoptosis: where do they meet? Apoptosis 19:555–566. https://doi.org/10.1007/s10495-014-0967-2
Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331:456–461. https://doi.org/10.1126/science.1196371
Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, Liu R, Zhong Q, Guan KL (2013) Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell 152:290–303. https://doi.org/10.1016/j.cell.2012.12.016
Chappell WH, Steelman LS, Long JM, Kempf RC, McCubrey JA (2011) Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget 2:135–164. https://doi.org/10.18632/oncotarget.240
Denisenko TV, Pivnyuk AD, Zhivotovsky B (2018) p53-Autophagy-Metastasis Link. Cancers (Basel) 10:148. https://doi.org/10.3390/cancers10050148
Fleming A, Noda T, Yoshimori T, Rubinsztein DC (2011) Chemical modulators of autophagy as biological probes and potential therapeutics. Nat Chem Biol 7:9–17. https://doi.org/10.1038/nchembio.500
Kornicka K, Kocherova I, Marycz K (2017) The effects of chosen plant extracts and compounds on mesenchymal stem cells-a bridge between molecular nutrition and regenerative medicine-concise review. Phytother Res 31:947–958. https://doi.org/10.1002/ptr.5812
Wang B, Lin Y, Hu Y, Shan W, Liu S, Xu Y, Zhang H, Cai S, Yu X, Cai Z, Huang H (2017) mTOR inhibition improves the immunomodulatory properties of human bone marrow mesenchymal stem cells by inducing COX-2 and PGE2. Stem Cell Res Ther 8:292. https://doi.org/10.1186/s13287-017-0744-6
Gao L, Cen S, Wang P, Xie Z, Liu Z, Deng W, Su H, Wu X, Wang S, Li J (2016) Autophagy improves the immunosuppression of CD4+ T cells by mesenchymal stem cells through transforming growth factor-β1. Stem Cells Transl Med 5:1496–1505. https://doi.org/10.5966/sctm.2015-0420
Gu Z, Wei T, Ji J (2016) Rapamycin reverses the senescent phenotype and improves immunoregulation of mesenchymal stem cells from MRL/lpr mice and systemic lupus erythematosus patients through inhibition of the mTOR signaling pathway. Aging 8:1102–1114. https://doi.org/10.18632/aging.100925
Dang S, Xu H, Xu C, Cai W, Li Q, Cheng Y, Jin M, Wang RX, Peng Y, Zhang Y, Wu C, He X, Wan B, Zhang Y (2014) Autophagy regulates the therapeutic potential of mesenchymal stem cells in experimental autoimmune encephalomyelitis. Autophagy 10:1301–1315. https://doi.org/10.4161/auto.28771
Wan Y, Zhuo N, Li Y, Zhao W, Jiang D (2017) Autophagy promotes osteogenic differentiation of human bone marrow mesenchymal stem cell derived from osteoporotic vertebrae. Biochem Biophys Res Commun 488:46–52. https://doi.org/10.1016/j.bbrc.2017.05.004
Lee JH, Jeong JK, Park SY (2018) AMPK activation mediated by hinokitiol inhibits adipogenic differentiation of mesenchymal stem cells through autophagy flux. Int J Endocrinol 2018:1–12. https://doi.org/10.1155/2018/2014192
Chen MW, Hu Y, Hou YH, Li MH, Chen MH, Mu CY, Tao BL, Zhu W, Luo Z, Cai KY (2019) Differentiation regulation of mesenchymal stem cells via autophagy induced by structurally-different silica based nanobiomaterials. J Mater Chem B 7:2657–2666. https://doi.org/10.1039/c9tb00040b
Li Y, Wang C, Zhang G, Wang X, Duan R, Gao H, Peng T, Teng J, Jia Y (2014) Role of autophagy and mTOR signaling in neural differentiation of bone marrow mesenchymal stem cells. Cell Biol Int 38:1337–1343. https://doi.org/10.1002/cbin.10320
Zhang GY, Jia YJ, Wang J, San-Song LI, Tang GH, Wang MM, Wang YW, Zhu DN (2017) Lithium chloride promotes neuronal differentiation of rat bone marrow mesenchymal stem cells by modulating autophagy. Chin J Pathophysiol 33:2128–2133 (in chinese). https://doi.org/10.3969/j.issn.1000-4718.2017.12.003
Park S, Choi Y, Jung N, Kim J, Oh S, Yu Y, Ahn JH, Jo I, Choi BO, Jung SC (2017) Autophagy induction in the skeletal myogenic differentiation of human tonsil-derived mesenchymal stem cells. Int J Mol Med 39:831–840. https://doi.org/10.3892/ijmm.2017.2898
Wang X, Lin Q, Zhang T, Wang X, Cheng K, Gao M, Xia P, Li X (2019) Low-intensity pulsed ultrasound promotes chondrogenesis of mesenchymal stem cells via regulation of autophagy. Stem Cell Res Ther 10:41–51. https://doi.org/10.1186/s13287-019-1142-z
Hou J, Han ZP, Jing YY, Yang X, Zhang SS, Sun K, Hao C, Meng Y, Yu FH, Liu XQ, Shi YF, Wu MC, Zhang L, Wei LX (2013) Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells. Cell Death Dis 4:e844. https://doi.org/10.1038/cddis.2013.338
Qian Z, Yue-Jin Y, Hong W, Qiu-Ting D, Tian-Jie W, Hai-Yan Q, Hui X (2012) Autophagy activation: a novel mechanism of atorvastatin to protect mesenchymal stem cells from hypoxia and serum deprivation via AMP-activated protein kinase/mammalian target of rapamycin pathway. Stem Cells Dev 21:1321. https://doi.org/10.1089/scd.2011.0684
Long W, Jing F, Yan-Shui L, Yun-Shan G, Bo G, Qi-Yue S, Bo-Yuan W, Li C, Liu Y, Jian L (2015) Glucocorticoids induce autophagy in rat bone marrow mesenchymal stem cells. Mol Med Rep 11:2711–2716. https://doi.org/10.3892/mmr.2014.3099
Wang L, Zhang HY, Gao B, Shi J, Huang Q, Han YH, Hu YQ, Lu WG, Zhao ZJ, Liu BH (2016) Tetramethylpyrazine protects against glucocorticoid-induced apoptosis by promoting autophagy in mesenchymal stem cells and improves bone mass in glucocorticoid-induced osteoporosis rats. J Orthop Transl 7:137–137. https://doi.org/10.1016/j.jot.2016.06.211
Yang CM, Huang YJ, Hsu SH (2015) Enhanced autophagy of adipose-derived stem cells grown on chitosan substrates. BioRes Open Access 4:89–96. https://doi.org/10.1089/biores.2014.0032
Liu Y, Wang N, Zhang S, Liang Q (2018) Autophagy protects bone marrow mesenchymal stem cells from palmitate-induced apoptosis through the ROS-JNK/p38 MAPK signaling pathways. Mol Med Rep 18:1485–1494. https://doi.org/10.3892/mmr.2018.9100
Choi JH, Lyu SY, Lee HJ, Jung J, Park WB, Kim GJ (2012) Korean mistletoe lectin regulates self-renewal of placenta-derived mesenchymal stem cells via autophagic mechanisms. Cell Prolif 45:420–429. https://doi.org/10.1111/j.1365-2184.2012.00839.x
Zhang M, Du Y, Lu R, Shu Y, Zhao W, Li Z, Zhang Y, Liu R, Yang T, Luo S (2016) Cholesterol retards senescence in bone marrow mesenchymal stem cells by modulating autophagy and ROS/p53/p21 Cip1/Waf1 pathway. Oxidative Med Cell Longev 2016:7524308. https://doi.org/10.1155/2016/7524308
Yun SP, Han YS, Lee JH, Kim SM, Lee SH (2017) Melatonin rescues mesenchymal stem cells from senescence induced by the uremic toxin p-cresol via inhibiting mTOR-dependent autophagy. Biomol Ther 26:389–398. https://doi.org/10.4062/biomolther.2017.071
Chang TC, Hsu MF, Wu KK (2015) High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS One 10:e0126537. https://doi.org/10.1371/journal.pone.0126537
Lv B, Hua T, Li F, Han J, Fang J, Xu L, Sun C, Zhang Z, Feng Z, Jiang X (2017) Hypoxia-inducible factor 1 α protects mesenchymal stem cells against oxygen-glucose deprivation-induced injury via autophagy induction and PI3K/AKT/mTOR signaling pathway. Am J Transl Res 9:2492–2499
Lee Y, Jung J, Cho KJ, Lee SK, Park JW, Oh IH, Kim GJ (2013) Increased SCF/c-kit by hypoxia promotes autophagy of human placental chorionic plate-derived mesenchymal stem cells via regulating the phosphorylation of mTOR. J Cell Biochem 114:79–88. https://doi.org/10.1002/jcb.24303
Agrahari G, Sah SK, Kim TY (2018) Superoxide dismutase 3 protects mesenchymal stem cells through enhanced autophagy and regulation of FoxO3a trafficking. BMB Rep 51:344–349. https://doi.org/10.5483/BMBRep.2018.51.7.078
Samuel H, Shi X, Johnson MH, Hamrick MW, Isales CM, Hill WD, Stan G (2013) Stromal cell-derived factor-1β mediates cell survival through enhancing autophagy in bone marrow-derived mesenchymal stem cells. PLoS One 8:e58207. https://doi.org/10.1371/journal.pone.0058207
Wei W, An Y, An Y, Fei D, Wang Q (2018) Activation of autophagy in periodontal ligament mesenchymal stem cells promotes angiogenesis in periodontitis. J Periodontol 89:718–727. https://doi.org/10.1002/JPER.17-0341
Liu J, Hao H, Huang H, Tong C, Ti D, Dong L, Chen D, Zhao Y, Liu H, Han W (2015) Hypoxia regulates the therapeutic potential of mesenchymal stem cells through enhanced autophagy. Int J Low Extrem Wounds 14:63–72. https://doi.org/10.1177/1534734615573660
Kim KW, Moon SJ, Park MJ, Kim BM, Kim EK, Lee SH, Lee EJ, Chung BH, Yang CW, Cho ML (2015) Optimization of adipose tissue-derived mesenchymal stem cells by rapamycin in a murine model of acute graft-versus-host disease. Stem Cell Res Ther 6:202. https://doi.org/10.1186/s13287-015-0197-8
Wang HY, Li C, Liu WH, Deng FM, Ma Y, Guo LN, Kong H, Hu KA, Liu Q, Wu J, Sun J, Liu YL (2020) Autophagy inhibition via Becn1 downregulation improves the mesenchymal stem cells antifibrotic potential in experimental liver fibrosis. J Cell Physiol 235:2722–2737. https://doi.org/10.1002/jcp.29176
Zheng Y, Lei Y, Hu C, Hu C (2016) p53 regulates autophagic activity in senescent rat mesenchymal stromal cells. Exp Gerontol 75:64–71. https://doi.org/10.1016/j.exger.2016.01.004
Pantovic A, Krstic A, Janjetovic K, Kocic J, Harhaji-Trajkovic L, Bugarski D, Trajkovic V (2013) Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells. Bone 52:524–531. https://doi.org/10.1016/j.bone.2012.10.024
Song BQ, Chi Y, Li X, Du WJ, Han ZB, Tian JJ, Li JJ, Chen F, Wu HH, Han LX, Lu SH, Zheng YZ, Han ZC (2015) Inhibition of notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway. Cell Physiol Biochem 36:1991–2002. https://doi.org/10.1159/000430167
Williams RS, Cheng L, Mudge AW, Harwood AJ (2002) A common mechanism of action for three mood-stabilizing drugs. Nature 417:292–295. https://doi.org/10.1038/417292a
Zou J, Yue XY, Zheng SC, Zhang G, Chang H, Liao YC, Zhang Y, Xue MQ, Qi Z (2014) Cholesterol modulates function of connexin 43 gap junction channel via PKC pathway in H9c2 cells. Biochim Biophys Acta 1838:2019–2025. https://doi.org/10.1016/j.bbamem.2014.04.016
Boyette LB, Creasey OA, Guzik L, Lozito T, Tuan RS (2014) Human bone marrow-derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem Cells Transl Med 3:241–254. https://doi.org/10.5966/sctm.2013-0079
Acknowledgements
The authors appreciate to the college of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China.
Author information
Authors and Affiliations
Contributions
JQ.D. and LJ.Z. wrote the manuscript. ZH.Z., CW.G. and XY.H. edited the manuscript. SM.Y., JL.D., SZ.C., ZH.R., ZC.Z. and LH.S. supervised this work. Jiaqiang Deng and Lijun Zhong contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
All the authors who have taken part in this study declared that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, J., Zhong, L., Zhou, Z. et al. Autophagy: a promising therapeutic target for improving mesenchymal stem cell biological functions. Mol Cell Biochem 476, 1135–1149 (2021). https://doi.org/10.1007/s11010-020-03978-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03978-2