Abstract
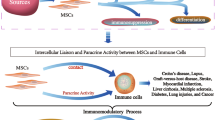
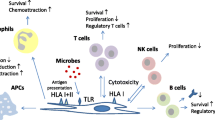
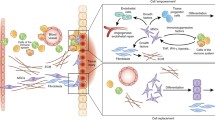
Mesenchymal stem cells (MSCs) are characterized as multipotent stromal cells with the capacity for both self-renewal and differentiation into mesodermal cell lineages. MSCs also have a fibroblast-like phenotype and can be isolated from several tissues. In recent years, researchers have found that MSCs secrete several soluble factors that exert immunosuppressive effects by modulating both innate (macrophages, dendritic and NK cells) and adaptive (B cells and CD4+ and CD8+ T cells) immune responses. This review summarizes the principal trophic factors that are related to immune regulation and secreted by MSCs under both autoimmune and inflammatory conditions. The understanding of mechanisms that regulate immunity in MSCs field is important for their future use as a novel cellular-based immunotherapy with clinical applications in several diseases.


Similar content being viewed by others
References
Luria, E. A., Panasyuk, A. F., & Friedenstein, A. Y. (1971). Fibroblast colony formation from monolayer cultures of blood cells. Transfusion, 11, 345–349.
da Silva Meirelles, L., Chagastelles, P. C., & Nardi, N. B. (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of Cell Science, 119, 2204–2213.
Kassem, M. (2004). Mesenchymal stem cells: Biological characteristics and potential clinical applications. Cloning and Stem Cells, 6, 369–374.
Dominici, M., Le Blanc, K., Mueller, I., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8, 315–317.
Horwitz, E. M., Le Blanc, K., Dominici, M., et al. (2005). Clarification of the nomenclature for MSC: The international society for cellular therapy position statement. Cytotherapy, 7, 393–395.
Si, Y. L., Zhao, Y. L., Hao, H. J., Fu, X. B., & Han, W. D. (2011). MSCs: Biological characteristics, clinical applications and their outstanding concerns. Ageing Research Reviews, 10, 93–103.
Bassi, E. J., Aita, C. A., & Camara, N. O. (2011). Immune regulatory properties of multipotent mesenchymal stromal cells: Where do we stand? World Journal Stem Cells, 3, 1–8.
Secco, M., Zucconi, E., Vieira, N. M., et al. (2008). Mesenchymal stem cells from umbilical cord: do not discard the cord! Neuromuscular Disorders, 18, 17–18.
Secco, M., Zucconi, E., Vieira, N. M., et al. (2008). Multipotent stem cells from umbilical cord: Cord is richer than blood! Stem Cells, 26, 146–150.
Panepucci, R. A., Siufi, J. L., Silva, W. A., Jr., et al. (2004). Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells, 22, 1263–1278.
Wu, K. H., Zhou, B., Lu, S. H., et al. (2007). In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. Journal of Cellular Biochemistry, 100, 608–616.
Ivanova-Todorova, E., Bochev, I., Mourdjeva, M., et al. (2009). Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunology Letters, 126, 37–42.
Jansen, B. J., Gilissen, C., Roelofs, H., et al. (2010). Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells and Development, 19, 481–490.
Devine, S. M., Cobbs, C., Jennings, M., Bartholomew, A., & Hoffman, R. (2003). Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood, 101, 2999–3001.
Le Blanc, K., Rasmusson, I., Gotherstrom, C., et al. (2004). Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scandinavian Journal of Immunology, 60, 307–315.
Aggarwal, S., & Pittenger, M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 105, 1815–1822.
Ghannam, S., Pene, J., Torcy-Moquet, G., Jorgensen, C., & Yssel, H. (2010). Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. Journal of Immunology, 185, 302–312.
Hemdan, N. Y., Birkenmeier, G., Wichmann, G., et al. (2010). Interleukin-17-producing T helper cells in autoimmunity. Autoimmunity Reviews, 9, 785–792.
Corcione, A., Benvenuto, F., Ferretti, E., et al. (2006). Human mesenchymal stem cells modulate B-cell functions. Blood, 107, 367–372.
Tabera, S., Perez-Simon, J. A., Diez-Campelo, M., et al. (2008). The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica, 93, 1301–1309.
Nauta, A. J., Kruisselbrink, A. B., Lurvink, E., Willemze, R., & Fibbe, W. E. (2006). Mesenchymal stem cells inhibit generation and function of both CD34+−derived and monocyte-derived dendritic cells. Journal of Immunology, 177, 2080–2087.
Khalil, N. (1999). TGF-beta: From latent to active. Microbes and Infection, 1, 1255–1263.
Rehman, J., Traktuev, D., Li, J., et al. (2004). Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation, 109, 1292–1298.
Tomic, S., Djokic, J., Vasilijic, S., et al. (2011). Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by toll-like receptor agonists. Stem Cells and Development, 20, 695–708.
English, K., Ryan, J. M., Tobin, L., Murphy, M. J., Barry, F. P., & Mahon, B. P. (2009). Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clinical and Experimental Immunology, 156, 149–160.
Patel, S. A., Meyer, J. R., Greco, S. J., Corcoran, K. E., Bryan, M., & Rameshwar, P. (2010). Mesenchymal stem cells protect breast cancer cells through regulatory T cells: Role of mesenchymal stem cell-derived TGF-beta. Journal of Immunology, 184, 5885–5894.
Nemeth, K., Keane-Myers, A., Brown, J. M., et al. (2010). Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proceedings of the National Academy of Sciences of the United States of America, 107, 5652–5657.
Nakamura, T. (1991). Structure and function of hepatocyte growth factor. Progress in Growth Factor Research, 3, 67–85.
Segura-Flores, A. A., Galvez-Gastelum, F. J., Alvarez-Rodriguez, A., & Armendariz-Borunda, J. (2004). Hepatocyte growth factor (HGF) and its therapeutic applications. Revista de Gastroenterologia de Mexico, 69, 243–250.
Di Nicola, M., Carlo-Stella, C., Magni, M., et al. (2002). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood, 99, 3838–3843.
Kang, J. W., Kang, K. S., Koo, H. C., Park, J. R., Choi, E. W., & Park, Y. H. (2008). Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells and Development, 17, 681–693.
Smith, W. L., Garavito, R. M., & DeWitt, D. L. (1996). Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and −2. Journal of Biological Chemistry, 271, 33157–33160.
Harris, S. G., Padilla, J., Koumas, L., Ray, D., & Phipps, R. P. (2002). Prostaglandins as modulators of immunity. Trends in Immunology, 23, 144–150.
Boniface, K., Bak-Jensen, K. S., Li, Y., et al. (2009). Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. The Journal of Experimental Medicine, 206, 535–548.
Hilkens, C. M., Snijders, A., Snijdewint, F. G., Wierenga, E. A., & Kapsenberg, M. L. (1996). Modulation of T-cell cytokine secretion by accessory cell-derived products. The European Respiratory Journal. Supplement, 22, 90s–94s.
Fedyk, E. R., Harris, S. G., Padilla, J., & Phipps, R. P. (1997). Prostaglandin receptors of the EP2 and EP4 subtypes regulate B lymphocyte activation and differentiation to IgE-secreting cells. Advances in Experimental Medicine and Biology, 433, 153–157.
Harizi, H., Juzan, M., Grosset, C., Rashedi, M., & Gualde, N. (2001). Dendritic cells issued in vitro from bone marrow produce PGE(2) that contributes to the immunomodulation induced by antigen-presenting cells. Cellular Immunology, 209, 19–28.
Kalinski, P., Hilkens, C. M., Snijders, A., Snijdewint, F. G., & Kapsenberg, M. L. (1997). Dendritic cells, obtained from peripheral blood precursors in the presence of PGE2, promote Th2 responses. Advances in Experimental Medicine and Biology, 417, 363–367.
Kleiveland, C. R., Kassem, M., & Lea, T. (2008). Human mesenchymal stem cell proliferation is regulated by PGE2 through differential activation of cAMP-dependent protein kinase isoforms. Experimental Cell Research, 314, 1831–1838.
English, K., Barry, F. P., Field-Corbett, C. P., & Mahon, B. P. (2007). IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunology Letters, 110, 91–100.
Chen, K., Wang, D., Du, W. T., et al. (2010). Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clinical Immunology, 135, 448–458.
Yanez, R., Oviedo, A., Aldea, M., Bueren, J. A., & Lamana, M. L. (2010). Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Experimental Cell Research, 316, 3109–3123.
Cui, L., Yin, S., Liu, W., Li, N., Zhang, W., & Cao, Y. (2007). Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Engineering, 13, 1185–1195.
Najar, M., Raicevic, G., Boufker, H. I., et al. (2010). Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: Combined comparison of adipose tissue, Wharton’s Jelly and bone marrow sources. Cellular Immunology, 264, 171–179.
Spaggiari, G. M., Abdelrazik, H., Becchetti, F., & Moretta, L. (2009). MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood, 113, 6576–6583.
Nemeth, K., Leelahavanichkul, A., Yuen, P. S., et al. (2009). Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature Medicine, 15, 42–49.
Yoshida, R., & Hayaishi, O. (1978). Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. Proceedings of the National Academy of Sciences of the United States of America, 75, 3998–4000.
Bianchi, M., Bertini, R., & Ghezzi, P. (1988). Induction of indoleamine dioxygenase by interferon in mice: A study with different recombinant interferons and various cytokines. Biochemical and Biophysical Research Communications, 152, 237–242.
DelaRosa, O., Lombardo, E., Beraza, A., et al. (2009). Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Engineering. Part A, 15, 2795–2806.
Tipnis, S., Viswanathan, C., & Majumdar, A. S. (2010). Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunology and Cell Biology, 88, 795–806.
Ge, W., Jiang, J., Arp, J., Liu, W., Garcia, B., & Wang, H. (2010). Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation, 90, 1312–1320.
Knowles, R. G., & Moncada, S. (1994). Nitric oxide synthases in mammals. Biochemical Journal, 298(Pt 2), 249–258.
Kleinert, H., Pautz, A., Linker, K., & Schwarz, P. M. (2004). Regulation of the expression of inducible nitric oxide synthase. European Journal of Pharmacology, 500, 255–266.
Bingisser, R. M., Tilbrook, P. A., Holt, P. G., & Kees, U. R. (1998). Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. Journal of Immunology, 160, 5729–5734.
Mais, A., Klein, T., Ullrich, V., Schudt, C., & Lauer, G. (2006). Prostanoid pattern and iNOS expression during chondrogenic differentiation of human mesenchymal stem cells. Journal of Cellular Biochemistry, 98, 798–809.
Sato, K., Ozaki, K., Oh, I., et al. (2007). Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood, 109, 228–234.
Oh, I., Ozaki, K., Sato, K., et al. (2007). Interferon-gamma and NF-kappaB mediate nitric oxide production by mesenchymal stromal cells. Biochemical and Biophysical Research Communications, 355, 956–962.
Ren, G., Zhang, L., Zhao, X., et al. (2008). Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell, 2, 141–150.
Ren, G., Su, J., Zhang, L., et al. (2009). Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells, 27, 1954–1962.
Ryter, S. W., & Choi, A. M. (2010). Heme oxygenase-1/carbon monoxide: novel therapeutic strategies in critical care medicine. Current Drug Targets, 11, 1485–1494.
Blancou, P., Tardif, V., Simon, T., et al. (2011). Immunoregulatory properties of heme oxygenase-1. Methods in Molecular Biology, 677, 247–268.
Chabannes, D., Hill, M., Merieau, E., et al. (2007). A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood, 110, 3691–3694.
Zarjou, A., Kim, J., Traylor, A. M., et al. (2011). Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. American Journal of Physiology. Renal Physiology, 300, F254–F262.
Fiorentino, D. F., Bond, M. W., & Mosmann, T. R. (1989). Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. Journal of Experimental Medicine, 170, 2081–2095.
Bogdan, C., Vodovotz, Y., & Nathan, C. (1991). Macrophage deactivation by interleukin 10. The Journal of Experimental Medicine, 174, 1549–1555.
Fiorentino, D. F., Zlotnik, A., Mosmann, T. R., Howard, M., & O’Garra, A. (1991). IL-10 inhibits cytokine production by activated macrophages. Journal of Immunology, 147, 3815–3822.
Moore, K. W., de Waal Malefyt, R., Coffman, R. L., & O’Garra, A. (2001). Interleukin-10 and the interleukin-10 receptor. Annual Review of Immunology, 19, 683–765.
Burdin, N., Van Kooten, C., Galibert, L., et al. (1995). Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. Journal of Immunology, 154, 2533–2544.
Macatonia, S. E., Doherty, T. M., Knight, S. C., & O’Garra, A. (1993). Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. Journal of Immunology, 150, 3755–3765.
Hedrich, C. M., & Bream, J. H. (2010). Cell type-specific regulation of IL-10 expression in inflammation and disease. Immunologic Research, 47, 185–206.
Yang, S. H., Park, M. J., Yoon, I. H., et al. (2009). Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Experimental & Molecular Medicine, 41, 315–324.
Rasmusson, I., Ringden, O., Sundberg, B., & Le Blanc, K. (2005). Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Experimental Cell Research, 305, 33–41.
Nasef, A., Chapel, A., Mazurier, C., et al. (2007). Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expression, 13, 217–226.
Burchfield, J. S., Iwasaki, M., Koyanagi, M., et al. (2008). Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circulation Research, 103, 203–211.
Razmkhah, M., Jaberipour, M., Erfani, N., Habibagahi, M., Talei, A. R., Ghaderi, A. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-beta1 and upregulate expression of regulatory molecules on T cells: Do they protect breast cancer cells from the immune response? Cell Immunol, 266, 116–122.
Kishimoto, T. (2006). Interleukin-6: Discovery of a pleiotropic cytokine. Arthritis Research and Therapy, 8(Suppl 2), S2.
Gabay, C. (2006). Interleukin-6 and chronic inflammation. Arthritis Research and Therapy, 8(Suppl 2), S3.
Djouad, F., Charbonnier, L. M., Bouffi, C., et al. (2007). Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells, 25, 2025–2032.
Najar, M., Rouas, R., Raicevic, G., et al. (2009). Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: The importance of low cell ratio and role of interleukin-6. Cytotherapy, 11, 570–583.
Xu, G., Zhang, Y., Zhang, L., Ren, G., & Shi, Y. (2007). The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochemical and Biophysical Research Communications, 361, 745–750.
Guo, Z., Zheng, C., Chen, Z., et al. (2009). Fetal BM-derived mesenchymal stem cells promote the expansion of human Th17 cells, but inhibit the production of Th1 cells. European Journal of Immunology, 39, 2840–2849.
Chen, B., Hu, J., Liao, L., et al. (2010). Flk-1+ mesenchymal stem cells aggravate collagen-induced arthritis by up-regulating interleukin-6. Clinical and Experimental Immunology, 159, 292–302.
Liu, X. J., Zhang, J. F., Sun, B., et al. (2009). Reciprocal effect of mesenchymal stem cell on experimental autoimmune encephalomyelitis is mediated by transforming growth factor-beta and interleukin-6. Clinical and Experimental Immunology, 158, 37–44.
Bai, L., Lennon, D. P., Eaton, V., et al. (2009). Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia, 57, 1192–1203.
Malemud, C. J. (2006). Matrix metalloproteinases (MMPs) in health and disease: An overview. Frontiers in Bioscience, 11, 1696–1701.
Parks, W. C., Wilson, C. L., & Lopez-Boado, Y. S. (2004). Matrix metalloproteinases as modulators of inflammation and innate immunity. Nature Reviews Immunology, 4, 617–629.
Ito, A., Mukaiyama, A., Itoh, Y., et al. (1996). Degradation of interleukin 1beta by matrix metalloproteinases. Journal of Biological Chemistry, 271, 14657–14660.
McQuibban, G. A., Gong, J. H., Tam, E. M., McCulloch, C. A., Clark-Lewis, I., & Overall, C. M. (2000). Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science, 289, 1202–1206.
Itoh, T., Matsuda, H., Tanioka, M., Kuwabara, K., Itohara, S., & Suzuki, R. (2002). The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. Journal of Immunology, 169, 2643–2647.
Ding, Y., Xu, D., Feng, G., Bushell, A., Muschel, R. J., & Wood, K. J. (2009). Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and −9. Diabetes, 58, 1797–1806.
Shen, Y., Winkler, I. G., Barbier, V., Sims, N. A., Hendy, J., & Levesque, J. P. (2010). Tissue inhibitor of metalloproteinase-3 (TIMP-3) regulates hematopoiesis and bone formation in vivo. PloS One, 5, e13086. doi:10.1371/journal.pone.0013086.
Lozito, T. P., & Tuan, R. S. (2011). Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. Journal of Cellular Physiology, 226, 385–396.
Tondreau, T., Meuleman, N., Stamatopoulos, B., et al. (2009). In vitro study of matrix metalloproteinase/tissue inhibitor of metalloproteinase production by mesenchymal stromal cells in response to inflammatory cytokines: the role of their migration in injured tissues. Cytotherapy, 11, 559–569.
Ries, C., Egea, V., Karow, M., Kolb, H., Jochum, M., & Neth, P. (2007). MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood, 109, 4055–4063.
Shu, T., Zeng, B., Ren, X., & Li, Y. (2010). HO-1 modified mesenchymal stem cells modulate MMPs/TIMPs system and adverse remodeling in infarcted myocardium. Tissue & Cell, 42, 217–222.
Carosella, E. D., HoWangYin, K. Y., Favier, B., & LeMaoult, J. (2008). HLA-G-dependent suppressor cells: Diverse by nature, function, and significance. Human Immunology, 69, 700–707.
Fainardi, E., Castellazzi, M., Stignani, M., et al. (2011). Emerging topics and new perspectives on HLA-G. Cellular and Molecular Life Sciences, 68, 433–451.
Rouas-Freiss, N., Goncalves, R. M., Menier, C., Dausset, J., & Carosella, E. D. (1997). Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proceedings of the National Academy of Sciences of the United States of America, 94, 11520–11525.
Lila, N., Amrein, C., Guillemain, R., et al. (2002). Human leukocyte antigen-G expression after heart transplantation is associated with a reduced incidence of rejection. Circulation, 105, 1949–1954.
LeMaoult, J., Zafaranloo, K., Le Danff, C., & Carosella, E. D. (2005). HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. The FASEB Journal, 19, 662–664.
Selmani, Z., Naji, A., Zidi, I., et al. (2008). Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells, 26, 212–222.
Davis, D. M. (2007). Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nature Reviews Immunology, 7, 238–243.
Deshmane, S. L., Kremlev, S., Amini, S., & Sawaya, B. E. (2009). Monocyte chemoattractant protein-1 (MCP-1): An overview. Journal of Interferon & Cytokine Research, 29, 313–326.
Rafei, M., Hsieh, J., Fortier, S., et al. (2008). Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood, 112, 4991–4998.
Luther, S. A., & Cyster, J. G. (2001). Chemokines as regulators of T cell differentiation. Nature Immunology, 2, 102–107.
Meirelles Lda, S., Fontes, A. M., Covas, D. T., & Caplan, A. I. (2009). Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine & Growth Factor Reviews, 20, 419–427.
Rafei, M., Campeau, P. M., Aguilar-Mahecha, A., et al. (2009). Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. Journal of Immunology, 182, 5994–6002.
Karnoub, A. E., Dash, A. B., Vo, A. P., et al. (2007). Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature, 449, 557–563.
Pinilla, S., Alt, E., Abdul Khalek, F. J., et al. (2009). Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Letters, 284, 80–85.
Soria, G., & Ben-Baruch, A. (2008). The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Letters, 267, 271–285.
Aghajanova, L. (2004). Leukemia inhibitory factor and human embryo implantation. Annals of the New York Academy of Sciences, 1034, 176–183.
Metcalf, D. (2003). The unsolved enigmas of leukemia inhibitory factor. Stem Cells, 21, 5–14.
Nasef, A., Mazurier, C., Bouchet, S., et al. (2008). Leukemia inhibitory factor: Role in human mesenchymal stem cells mediated immunosuppression. Cellular Immunology, 253, 16–22.
Najar, M., Raicevic, G., Boufker, H. I., et al. (2010). Adipose-tissue-derived and Wharton’s jelly-derived mesenchymal stromal cells suppress lymphocyte responses by secreting leukemia inhibitory factor. Tissue Engineering. Part A, 16, 3537–3546.
Acknowledgments
This study was supported by grants 08/55447-1, 09/51649-1, 10/12295-7, 10/16213-5 and 07/07139-3 from the State of Sao Paulo Foundation for Research Support (FAPESP), Brazilian Council of Scientific and Technologic Development (470533/2007-2, CNPq/DECIT/MS) and Complex Fluids INCT.
Conflicts of interest
The authors declare no potential conflicts of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bassi, Ê.J., de Almeida, D.C., Moraes-Vieira, P.M.M. et al. Exploring the Role of Soluble Factors Associated with Immune Regulatory Properties of Mesenchymal Stem Cells. Stem Cell Rev and Rep 8, 329–342 (2012). https://doi.org/10.1007/s12015-011-9311-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-011-9311-1