Abstract
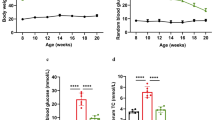
The purpose of the study was to observe changes in endoplasmic reticulum stress (ERS)- and autophagy-related proteins in gastric smooth muscle tissues of diabetic rats with gastroparesis, investigate the effect of insulin-like growth factor 1 (IGF-1) on ERS and autophagy in rat gastric smooth muscle cells cultured under different glucose concentrations, and explore the influence of IGF-1 on development of diabetic gastroparesis (DGP). After establishing a rat model of DGP, rats were divided into normal control (NC) and 6-week diabetic model (DM6W) groups. Expression of ERS-related and autophagy-related proteins was detected by western blot analysis and immunofluorescence assay in rat gastric smooth muscle tissue and in vitro-cultured rat gastric smooth muscle cells exposed to different glucose concentrations and treatment with IGF-1 for 24 or 48 h. Changes in glucose-regulated-protein-78 (GRP78), growth arrest and DNA damage-inducible gene 153 (CHOP), and microtubule-associated protein 1A/1B light chain 3B (LC3) expression levels were detected by western blot analysis, and GRP78 and LC3 expression were examined by confocal laser-scanning microscopy. In vivo expression levels of GRP78, CHOP, and LC3 were significantly higher in the DM6W group compared with the NC group (p < 0.001). Twenty-four hours after cells were cultured at different glucose concentrations in vitro, expression of GRP78, CHOP, and LC3II/I was significantly higher in the high glucose-treated group compared with the normal glucose group (p < 0.05). After IGF-1 intervention, CHOP and GRP78 expression were significantly higher in the normal glucose + IGF-1 group compared with the normal glucose group (p < 0.01), while no significant difference was found between high glucose and high glucose + IGF-1 groups. LC3II/I expression was significantly lower in the normal glucose + IGF-1 group compared with the normal glucose group, and was significantly lower in the high glucose and high glucose + IGF-1 groups (p < 0.05). After 48 h of culture, CHOP expression was significantly higher and LC3II/I expression was significantly lower in the high glucose group compared with the normal glucose group (p < 0.05), but no significant change in GRP78 expression was observed between these two groups. After IGF-1 intervention, there was no difference in CHOP or GRP78 expression between normal glucose + IGF-1 and normal glucose groups. However, CHOP and GRP78 expression were significantly lower in the high glucose + IGF-1 group compared with the high glucose group (p < 0.05). There was no significant difference in LC3II/I expression between normal glucose + IGF-1 and normal glucose groups, or high glucose + IGF-1 and high glucose groups. Results of confocal laser-scanning microscopy showed significantly lower expression of LC3II/I in the high glucose + IGF-1 group compared with the high glucose group (p < 0.05). ERS and autophagy were involved in the occurrence of DGP. IGF-1 exerted an inhibitory effect on ERS in rat gastric smooth muscle cells cultured under high glucose conditions, and this inhibitory effect increased with time. IGF-1 inhibited the level of autophagy in rat gastric smooth muscle cells cultured under high glucose conditions at early stages, which may be achieved through inhibition of ERS.




Similar content being viewed by others
References
Phillips LK, Deane AM, Jones KL, Rayner CK, Horowitz M (2015) Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol 11(2):112–128
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529
Schröder M, Sutcliffe L (2010) Consequences of stress in the secretory pathway: the ER stress response and its role in the metabolic syndrome. Methods Mol Biol 648:43–62
Zhong JT, Xu Y, Yi HW, Su J, Yu HM, Xiang XY, Li XN, Zhang ZC, Sun LK (2012) The BH3 mimetic S1 induces autophagy through ER stress and disruption of Bcl-2/Beclin 1 interaction in human glioma U251 cells. Cancer Lett 323(2):180–187
Carroll PV (2001) Treatment with growth hormone and insulin-like growth factor-I in critical illness. Best Pract Res Clin Endocrinol Metab 15(4):435–451
Xu DY, Liu L, Cai YL, Li XL, Qiu ZX, Jin Z, Xu WX (2010) Natriuretic peptide-dependent cGMP signal pathway potentiated the relaxation of gastric smooth muscle in streptozotocin-induced diabetic rats. Dig Dis Sci 55(3):589–595
Cai YL, Xu DY, Li XL, Qiu ZX, Jin Z, Xu WX (2009) C-type natriuretic-peptide-potentiated relaxation response of gastric smooth muscle in streptozotocin-induced diabetic rats. World J Gastroenterol 15(17):2125–2131
Zhang MH, Jiang JZ, Cai YL, Piao LH, Jin Z (2017) Significance of dynamic changes in gastric smooth muscle cell apoptosis, PI3K-AKT-mTOR and AMPK-mTOR signaling in a rat model of diabetic gastroparesis. Mol Med Rep 16(2):1530–1536
Cnop M, Foufelle F, Velloso LA (2012) Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med 18(1):59–68
Hummasti S, Hotamisligil GS (2010) Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ Res 107(5):579–591
Zhang YW, Wang X, Ren X, Zhang M. Involvement of glucose-regulated protein 78 and spliced X-box binding protein 1 in the protective effect of gliclazide in diabetic nephropathy. Diabetes Res Clin Pract (2017). https://doi.org/10.1016/j.diabres.2017.04.019
Zhou Y, Wu W (2017) The sodium-glucose co-transporter 2 inhibitor, empagliflozin, protects against diabetic cardiomyopathy by inhibition of the endoplasmic reticulum stress pathway. Cell Physiol Biochem 41(6):2503–2512
Chen D, Wang Y, Chin ER (2015) Activation of the endoplasmic reticulum stress response in skeletal muscle of G93A* SOD1 amyotrophic lateral sclerosis mice. Front Cell Neurosci 9:170
Roohbakhsh A, Shirani K, Karimi G (2016) Methamphetamine-induced toxicity: the role of autophagy? Chem Biol Interact 260:163–167
Liu Y, Zhang J, Wang Y, Zeng X (2017) Apelin involved in progression of diabetic nephropathy by inhibiting autophagy in podocytes. Cell Death Dis 8(8):e3006
Yang F, Zhang L, Gao Z, Sun X, Yu M, Dong S, Wu J, Zhao Y, Xu C, Zhang W, Lu F (2017) Exogenous H2S protects against diabetic cardiomyopathy by activating autophagy via the AMPK/mTOR pathway. Cell Physiol Biochem 43(3):1168–1187
Chen X, Fu XS, Li CP, Zhao HX (2014) ER stress and ER stress-induced apoptosis are activated in gastric SMCs in diabetic rats. World J Gastroenterol 20:8260–8267
Kim Y, Li E, Park S (2012) Insulin-like growth factor-1 inhibits 6-hydroxydopamine-mediated endoplasmic reticulum stress-induced apoptosis via regulation of heme oxygenase-1 and Nrf2 expression in PC12 cells. Int J Neurosci 122(11):641–649
Nazli SA, Loeser RF, Chubinskaya S, Willey JS, Yammani RR (2017) High fat-diet and saturated fatty acid palmitate inhibits IGF-1 function in chondrocytes. Osteoarthr Cartil 25(9):1516–1521
Pfaffenbach KT, Pong M, Morgan TE, Wang H, Ott K, Zhou B, Longo VD, Lee AS (2012) GRP78/BiP is a novel downstream target of IGF-1 receptor mediated signaling. J Cell Physiol 227(12):3803–3811
Zhao Y, Zhang L, Qiao Y, Zhou X, Wu G, Wang L, Peng Y, Dong X, Huang H, Si L, Zhang X, Zhang L, Li J, Wang W, Zhou L, Gao X (2013) Heme oxygenase-1 prevents cardiac dysfunction in streptozotocin-diabetic mice by reducing inflammation, oxidative stress, apoptosis and enhancing autophagy. PLoS ONE 8(9):e75927
Fang L, Zhou Y, Cao H, Wen P, Jiang L, He W, Dai C, Yang J (2013) Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS ONE 8(4):e60546
Wang HL, Yu KJ (2015) Sequential blood purification therapy for critical patients with hyperlipidemic severe acute pancreatitis. World J Gastroenterol 21(20):6304–6309
Acknowledgements
This study was financially supported by Grants from the National Natural Science Foundation of China (Grant Nos. 81360070 and 81560142).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no actual or potential conflict of interest in relation to this article.
Rights and permissions
About this article
Cite this article
Fang, Xs., Zhang, Mh., Guo, Jy. et al. Effects of insulin-like growth factor-1 on endoplasmic reticulum stress and autophagy in rat gastric smooth muscle cells cultured at different glucose concentrations in vitro. Mol Cell Biochem 451, 11–20 (2019). https://doi.org/10.1007/s11010-018-3388-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3388-7