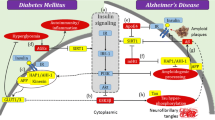
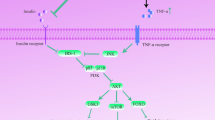
Abstract
Insulin resistance is common in type 2 diabetes mellitus (T2DM), neurodegenerative diseases, cardiovascular diseases, kidney diseases, and polycystic ovary syndrome. Impairment in insulin signaling pathways, such as the PI3K/Akt/mTOR pathway, would lead to insulin resistance. It might induce the synthesis and deposition of advanced glycation end products (AGEs), reactive oxygen species, and reactive nitrogen species, resulting in stress, protein misfolding, protein accumulation, mitochondrial dysfunction, reticulum function, and metabolic syndrome dysregulation, inflammation, and apoptosis. It plays a huge role in various neurodegenerative diseases like Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, and Amyloid lateral sclerosis. In this review, we intend to focus on the possible effect of insulin resistance in the progression of neurodegeneration via the impaired P13K/Akt/mTOR signaling pathway, AGEs, and receptors for AGEs.






Similar content being viewed by others
Data Availability
Data sharing is not applicable for this study as no datasets are genarated or analyzed in the current study.
Abbreviations
- 4E BP1:
-
Eukaryotic translation initiation factor 4E (elF4E)-binding protein 1
- AD:
-
Alzheimer’s disease
- AGEs:
-
Advances glycation end products
- ALS:
-
Amyloid lateral sclerosis
- AKT:
-
AK strain transforming
- AMPK:
-
AMP-activated protein kinase
- APP:
-
Amyloid precursor protein
- APS:
-
Adaptor protein with Pleckstrin homology and Src homology 2 domains
- ATP:
-
Adenosine triphosphate
- ATG:
-
Autophagy-related gene
- Bax:
-
Bcl-2 associated X protein
- BBB:
-
Blood–Brain Barrier
- BNIP 3:
-
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3
- CML:
-
Nε-carboxy-methyl-lysine
- c-Myc:
-
Master Regulator of Cell Cycle Entry and Proliferative Metabolism
- Cpd38:
-
6-(2,4-difluorophenoxy)-5-((ethylmethyl)pyridine-3-yl)-8-methylpyrrolo[1,2-a] pyrazin-1(2H)-one
- CREB:
-
cAMP-responsive element-binding protein
- CVD:
-
Cardiovascular diseases
- DEPTOR:
-
DEP domain-containing interacting protein
- DPP4:
-
Dipeptidyl-peptidase 4
- DM:
-
Diabetes mellitus
- ERK:
-
Extracellular signal-regulated kinase
- FOX O:
-
Forehead box O transcription factor
- FRK:
-
Fyn-related kinase
- FUS/TLS:
-
Fused in sarcoma/translocated in liposarcoma
- GLP 1:
-
Glucagon-like polypeptide 1
- GABA:
-
Gama-aminobutyric acid
- GLUT:
-
Glucose transporter
- GPCR:
-
G protein-coupled receptor
- GRB:
-
Growth factor receptor-bound protein
- GSK:
-
Glycogen synthase kinase
- HD:
-
Huntington’s disease
- HIF1a:
-
Hypoxia-inducible Factor-1a
- HTT:
-
Huntington protein
- IGF:
-
Insulin growth factor
- IL:
-
Interleukin
- IR:
-
Insulin receptor
- IRS:
-
Insulin receptor substrate
- JNK:
-
Jun kinase
- MAPK:
-
Mitogen-activated protein kinase
- MELAS:
-
Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like episodes
- MLST8:
-
Mammalian lethal with SEC18 protein 8
- mSIN- mLST 1- mTOR:
-
Mechanistic/mammalian target of rapamycin
- NADPH:
-
Mitogen-activated protein kinase
- NF κb:
-
Nuclear factor kappa-light chain-enhancer of activated B cells
- Nrf:
-
Nuclear factor κ-light-chain-enhancer of activated B cell
- NMDA:
-
N-methyl-D-aspartate
- PD:
-
Parkinson’s disease
- PDK:
-
Phosphoinositide-dependent Protein Kinase
- Pkc:
-
Protein Kinase C
- PI3K:
-
Phosphatidyl inositol 3 phosphate kinase
- PIP3:
-
Phosphatidyl inositol 3,4,5 triphosphate
- PINK:
-
PTEN putative kinase 1
- PKB:
-
Protein kinase B
- PTEN:
-
Phosphatase and tensin homolog
- PTP1B:
-
Protein-tyrosine Phosphatase 1B
- PP-242:
-
mTOR inhibitor
- PPAR:
-
Peroxisome proliferator-activated receptor
- PRAS 8:
-
Protease-activated receptors 8
- RTK:
-
Receptor tyrosine kinase
- RAGE:
-
Receptor for AGEs
- RAPTOR:
-
Regulatory associated protein of mTOR
- RHEB:
-
Ras homologous enriched in the brain
- RICTOR:
-
Rapamycin-insensitive companion of mTOR
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- RXR:
-
Retinoid transcription factor
- SREBP1:
-
Sterol regulatory element-binding transcription factor 1
- SGK:
-
Serine/threonine-protein kinase
- SR:
-
Scavenger receptor
- S6K:
-
Ribosomal s6 kinase
- SOXS3:
-
Suppressor of cytokine signaling 3
- SOD1:
-
Superoxide dismutase 1
- STAT:
-
Signal transducer and activator of transcription
- SHcA:
-
SHC transforming protein 1
- TDP-43:
-
TAR DNA-binding protein 43
- TSC:
-
Tuberous sclerosis proteins
- TNF:
-
Tumor necrosis factor
- VEGF:
-
Vascular endothelial growth factor
- Wnt:
-
Wingless-related integration site
References
Dai B, Wu Q, Zeng C, Zhang J, Cao L, Xiao Z, Yang M (2016) The effect of Liuwei Dihuang Decoction on PI3K/Akt signaling pathway in liver of type 2 Diabetes Mellitus (T2DM) rats with insulin resistance. J Ethnopharmacol 192:382–389. https://doi.org/10.1016/j.jep.2016.07.024
He CJ, Ma LQ, Iqbal MS, Huang XJ, Li J, Yang GZ, Ihsan A (2020) Veratrilla Baillonii Franch exerts anti-diabetic activity and improves liver injury through IRS/PI3K/AKT signaling pathways in Type 2 Diabetic Db/Db mice. J Funct Foods 75:104204. https://doi.org/10.1016/j.jff.2020.104204
Yang L, Wang H, Liu L, Xie A (2018) The role of insulin/IGF-1/PI3K/Akt/GSK3β signaling in Parkinson’s disease dementia. Front Neurosci 12:1–8. https://doi.org/10.3389/fnins.2018.00073
Aviles-Olmos I, Limousin P, Lees A, Foltynie T (2013) Parkinson’s Disease, insulin resistance and novel agents of neuroprotection. Brain 136:374–384. https://doi.org/10.1093/brain/aws009
Yang YW, Hsieh TF, Li CI, Liu CS, Lin WY, Chiang JH, Li TC, Lin CC (2017) Increased risk of Parkinson disease with diabetes mellitus in a population-based study. Medicine (United States). https://doi.org/10.1097/MD.0000000000005921
Chu F, Li K, Li X, Xu L, Huang J, Yang Z (2021) Graphene oxide ameliorates the cognitive impairment through inhibiting PI3K/Akt/MTOR pathway to induce autophagy in AD mouse model. Neurochem Res 46:309–325. https://doi.org/10.1007/s11064-020-03167-z
Barbier P, Zejneli O, Martinho M, Lasorsa A, Belle V, Smet-Nocca C, Tsvetkov PO, Devred F, Landrieu I (2019) Role of Tau as a microtubule-associated protein: structural and functional aspects. Front Aging Neurosci 10:1–14. https://doi.org/10.3389/fnagi.2019.00204
Brothers HM, Gosztyla ML, Robinson SR (2018) The physiological roles of amyloid-β peptide hint at new ways to treat Alzheimer’s Disease. Front Aging Neurosci 10:1–16. https://doi.org/10.3389/fnagi.2018.00118
Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A (2014) Role of advanced glycation end products in cellular signaling. Redox Biol 2:411–429. https://doi.org/10.1016/j.redox.2013.12.016
Zhao G, Zhang X, Wang H, Chen Z (2020) Beta carotene protects H9c2 cardiomyocytes from advanced glycation end product-induced endoplasmic reticulum stress, apoptosis, and autophagy via the PI3K / Akt / MTOR signaling pathway. Ann Transl Med 8:1–13. https://doi.org/10.21037/atm-20-3768
Sharma A, Weber D, Raupbach J, Dakal TC, Fließbach K, Ramirez A, Grune T, Wüllner U (2020) Advanced glycation end products and protein carbonyl levels in plasma reveal sex-specific differences in Parkinson’s and Alzheimer’s disease. Redox Biol 34:101546. https://doi.org/10.1016/j.redox.2020.101546
Kim Y, Kim C, Son SM, Song H, Hong HS, Han SH, Mook-Jung I (2016) The novel RAGE Interactor PRAK is associated with autophagy signaling in Alzheimer’s disease pathogenesis. Mol Neurodegener 11:1–11. https://doi.org/10.1186/s13024-016-0068-5
Adamopoulos C, Farmaki E, Spilioti E, Kiaris H, Piperi C (2013) Advanced glycation end-products induce endoplasmic reticulum stress in human aortic endothelial cells. Clin Chem Lab Med. https://doi.org/10.1515/cclm-2012-0826
Xu F, Na L, Li Y, Chen L (2020) Roles of the PI3K/AKT/MTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci 10:1–12. https://doi.org/10.1186/s13578-020-00416-0
Yan J, Wang C, Jin Y, Meng Q, Liu Q, Liu Z, Liu K, Sun H (2018) Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol Res 130:466–480. https://doi.org/10.1016/j.phrs.2017.12.026
Dos Santos JM, Tewari S, Mendes RH (2019) The role of oxidative stress in the development of diabetes mellitus and its complications. J Diabetes Res 2019:10–12. https://doi.org/10.1155/2019/4189813
Wang J, Yang X, Zhang J (2016) Bridges between mitochondrial oxidative stress, ER stress and MTOR signaling in pancreatic β cells. Cell Signal 28:1099–1104. https://doi.org/10.1016/j.cellsig.2016.05.007
Jere SW, Houreld NN, Abrahamse H (2019) Role of the PI3K/AKT (MTOR and GSK3β) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev 50:52–59. https://doi.org/10.1016/j.cytogfr.2019.03.001
Hassanpour M, Rezabakhsh A, Rahbarghazi R, Nourazarian A, Nouri M, Avci ÇB, Ghaderi S, Alidadyani N, Bagca BG, Bagheri HS (2017) Functional convergence of Akt protein with VEGFR-1 in human endothelial progenitor cells exposed to sera from patient with type 2 diabetes mellitus. Microvasc Res 114:101–113. https://doi.org/10.1016/j.mvr.2017.07.002
Bathina S, Gundala NKV, Rhenghachar P, Polavarapu S, Hari AD, Sadananda M, Das UN (2020) Resolvin D1 ameliorates nicotinamide-streptozotocin-induced type 2 diabetes mellitus by its anti-inflammatory action and modulating PI3K/Akt/MTOR pathway in the brain. Arch Med Res 51:492–503. https://doi.org/10.1016/j.arcmed.2020.05.002
Di Tu Q, Jin J, Hu X, Ren Y, Zhao L, He Q (2020) Curcumin improves the renal autophagy in rat experimental membranous nephropathy via regulating the PI3K/AKT/MTOR and Nrf2/HO-1 signaling pathways. BioMed Res Int. https://doi.org/10.1155/2020/7069052
Villalobos-Labra R, Silva L, Subiabre M, Araos J, Salsoso R, Fuenzalida B, Sáez T, Toledo F, González M, Quezada C, Pardo F, Chiarello DI, Leiva A, Sobrevia L (2017) Akt/MTOR role in human foetoplacental vascular insulin resistance in diseases of pregnancy. J Diabetes Res. https://doi.org/10.1155/2017/5947859
Mardilovich K, Pankratz SL, Shaw LM (2009) Expression and function of the insulin receptor substrate proteins in cancer. Cell Commun Signal 7:1–15. https://doi.org/10.1186/1478-811X-7-14
Tumminia A, Vinciguerra F, Parisi M, Frittitta L (2018) Type 2 diabetes mellitus and Alzheimer’s disease: role of insulin signalling and therapeutic implications. Int J Mol Sci. https://doi.org/10.3390/ijms19113306
Bao S, Wu YL, Wang X, Han S, Cho SB, Ao W, Nan JX (2020) Agriophyllum oligosaccharides ameliorate hepatic injury in type 2 diabetic Db/Db mice targeting INS-R/IRS-2/PI3K/AKT/PPAR-γ/Glut4 signal pathway. J Ethnopharmacol 257:112863. https://doi.org/10.1016/j.jep.2020.112863
Rabiee A, Krüger M, Ardenkjær-Larsen J, Kahn CR, Emanuelli B (2018) Distinct signalling properties of insulin receptor substrate (IRS)-1 and IRS-2 in mediating insulin/IGF-1 action. Cell Signal 47:1–15. https://doi.org/10.1016/j.cellsig.2018.03.003
Zoncu R, Efeyan A, Sabatini D (2012) MTOR: from growth signal integration to cancer. Diabetes Ageing. 12:21–35. https://doi.org/10.1038/nrm3025.mTOR
Kubota T, Kubota N, Kadowaki T (2017) Imbalanced insulin actions in obesity and type 2 diabetes : key mouse models of insulin signaling pathway. Cell Metab 25:797–810. https://doi.org/10.1016/j.cmet.2017.03.004
Wang Q, Cheng XL, Zhang DY, Gao XJ, Zhou L, Qin XY, Xie GY, Liu K, Qin Y, Liu BL, Qin MJ (2013) Tectorigenin attenuates palmitate-induced endothelial insulin resistance via targeting ROS-associated inflammation and IRS-1 pathway. PLoS One. https://doi.org/10.1371/journal.pone.0066417
Deyev IE, Sohet F, Vassilenko KP, Serova OV, Nadezhda V, Zozulya SA, Burova EB, Houillier P, Rzhevsky DI, Berchatova AA, Murashev AN, Chugunov AO, Roman G, Nikol NN, Bertelli E, Eladari D, Alexander G (2012) Insulin receptor-related receptor as an extracellular alkali sensor. Cell Metab. 13:679–689. https://doi.org/10.1016/j.cmet.2011.03.022.Insulin
Boura-Halfon S, Zick Y (2009) Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. https://doi.org/10.1152/ajpendo.90437.2008
Gao S, Guo Q, Qin C, Shang R, Zhang Z (2017) Sea Buckthorn fruit oil extract alleviates insulin resistance through the PI3K/Akt signaling pathway in type 2 diabetes mellitus cells and rats. J Agric Food Chem 65:1328–1336. https://doi.org/10.1021/acs.jafc.6b04682
O’Neill C (2013) PI3-Kinase/Akt/MTOR signaling: impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp Gerontol 48:647–653. https://doi.org/10.1016/j.exger.2013.02.025
Geering B, Cutillas PR, Nock G, Gharbi SI, Vanhaesebroeck B (2007) Class IA phosphoinositide 3-kinases are obligate P85–P110 heterodimers. Proc Natl Acad Sci USA 104:7809–7814. https://doi.org/10.1073/pnas.0700373104
Xie Y, Shi X, Sheng K, Han G, Li W, Zhao Q, Jiang B, Feng J, Li J, Gu Y (2019) PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (review). Mol Med Reports 19:783–791. https://doi.org/10.3892/mmr.2018.9713
Fan Y, He Z, Wang W, Li J, Hu A, Li L, Yan L, Li Z, Yin Q (2018) Tangganjian decoction ameliorates type 2 diabetes mellitus and nonalcoholic fatty liver disease in rats by activating the IRS/PI3K/AKT signaling pathway. Biomed Pharmacother 106:733–737. https://doi.org/10.1016/j.biopha.2018.06.089
Brachmann SM, Ueki K, Engelman JA, Kahn RC, Cantley LC (2005) Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Exp Gerontol 25:1596–1607. https://doi.org/10.1128/MCB.25.5.1596
Gupta A, Dey CS, Chernoff J (2009) PTEN, a widely known negative regulator of insulin / PI3K signaling Positively Regulates Neuronal Insulin Resistance. Mol Biol Cell. https://doi.org/10.1091/mbc.E12-05-0337
Dibble CC, Cantely LC (2015) Regulation of MTORC1 by PI3K signaling. Trends Cell Biol 25:545–555. https://doi.org/10.1016/j.tcb.2015.06.002.Regulation
Ersahin T, Tuncbag N, Cetin-Atalay R (2015) The PI3K/AKT/MTOR interactive pathway. Mol BioSyst 11:1946–1954. https://doi.org/10.1039/c5mb00101c
Bathina S, Das UN (2018) Dysregulation of PI3K-Akt-MTOR pathway in brain of streptozotocin-induced type 2 diabetes mellitus in Wistar rats. Lipids Health Dis 17:1–11. https://doi.org/10.1186/s12944-018-0809-2
Sudhahar V, Okur MN, Bagi Z, Bryan JPO, Hay N, Makino A, Patel VS, Phillips SA, Stepp D, Fukai T, Genetics M, Veterans B, Medical A, Veterans N, Medical A (2019) Akt2 stabilizes ATP7A, a Cu transporter for SOD3, in vascular smooth muscles: novel mechanism to limit endothelial dysfunction in Type2 diabetes. Arterioscler Thromb Vasc Biol. 38:529–541. https://doi.org/10.1161/ATVBAHA.117.309819.Akt2
Furlong RM (2019) The Parkinson’s gene PINK1 activates Akt via PINK1 kinase-dependent regulation of the phospholipid PI(3,4,5) P3. J Cell Sci 3:132. https://doi.org/10.1242/jcs.233221
Bian C, Bai B, Gao Q, Li S, Zhao Y (2019) 17β-estradiol regulates glucose metabolism and insulin secretion in rat islet β cells through GPER and Akt/MTOR/GLUT2 pathway. Front Endocrinol 10:1–12. https://doi.org/10.3389/fendo.2019.00531
Karki R, Tom A, Hofmann-Apitius M (2017) Comorbidity analysis between Alzheimer’s disease and type 2 diabetes mellitus ( T2DM ) based on shared pathways and the role of T2DM drugs. J Alzheimers Dis 60:721–731. https://doi.org/10.3233/JAD-170440
Tuohetaerbaike B, Zhang Y, Tian Y, nan Zhang N, Kang J, Mao X, Zhang Y, Li X (2020) Pancreas protective effects of urolithin a on type 2 diabetic mice induced by high fat and streptozotocin via regulating autophagy and AKT/MTOR signaling pathway. J Ethnopharmacol. https://doi.org/10.1016/j.jep.2019.112479
Rai SN, Dilnashin H, Birla H, Singh S. Sen, Zahra W, Rathore AS, Singh BK, Singh SP (2019) The role of PI3K/Akt and ERK in neurodegenerative disorders. Neurotox Res 35:775–795. https://doi.org/10.1007/s12640-019-0003-y
Wang Y, Duan W, Wang W, Wen D, Liu Y, Liu Y, Li Z, Hu H, Lin H, Cui C, Li D, Dong H (2016) ScAAV9-VEGF prolongs the survival of transgenic ALS mice by promoting activation of M2 microglia and the PI3K/Akt pathway. Brain Res. https://doi.org/10.1016/j.brainres.2016.06.043
Tuo Y, Xiang M (2019) MTOR: a double-edged sword for diabetes. J Leukocyte Biol 106:385–395. https://doi.org/10.1002/JLB.3MR0317-095RR
Katta A, Kakarla S, Wu M, Paturi S, Gadde MK, Arvapalli R, Kolli M, Rice KM, Blough ER (2009) Altered regulation of contraction-induced Akt/MTOR/P70S6k pathway signaling in skeletal muscle of the Obese Zucker Rat. Exp Diabetes Res 2009:384683. https://doi.org/10.1155/2009/384683
Muthukumaran P, Thiyagarajan G, Arun Babu R, Lakshmi BS (2018) Raffinose from Costus speciosus attenuates lipid synthesis through modulation of PPARs/SREBP1c and improves insulin sensitivity through PI3K/AKT. Chemico-Biol Interact 284:80–89. https://doi.org/10.1016/j.cbi.2018.02.011
Lu X, Paliogiannis P, Calvisi DF, Chen X (2021) Role of the mammalian target of rapamycin pathway in liver cancer: from molecular genetics to targeted therapies. Hepatology 73:49–61. https://doi.org/10.1002/hep.31310
Zheng G, Wang L, Li X, Niu X, Xu G, Lv P (2021) Rapamycin alleviates cognitive impairment in murine vascular dementia: the enhancement of mitophagy by PI3K/AKT/MTOR axis. Tissue Cell 69:101481. https://doi.org/10.1016/j.tice.2020.101481
Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X (2019) Targeting PI3K in cancer: mechanisms and advances in clinical trials 06 biological sciences 0601 biochemistry and cell biology. Mol Cancer 18:1–28
Kezic A, Popovic L, Lalic K (2018) MTOR inhibitor therapy and metabolic consequences: where do we stand? Oxid Med Cell Longev. https://doi.org/10.1155/2018/2640342
Koliaki C, Liatis S, Dalamaga M, Kokkinos A (2020) the implication of gut hormones in the regulation of energy homeostasis and their role in the pathophysiology of obesity. Curr Obesity Reports 9:255–271. https://doi.org/10.1007/s13679-020-00396-9
Wilcox G (2005) Insulin and insulin resistance. Aliment Pharmacol Ther Suppl 22:61–63. https://doi.org/10.1111/j.1365-2036.2005.02599.x
Peterson CT, Vaughn AR, Sharma V, Chopra D, Mills PJ, Peterson SN, Sivamani RK (2018) Effects of turmeric and curcumin dietary supplementation on human gut microbiota: a double-blind, randomized, placebo-controlled pilot study. J Evid Based Integr Med 23:1–8. https://doi.org/10.1177/2515690X18790725
Bryan MR, Nordham KD, Rose DIR, Joshi P, Foshage AM, Nitin R, Michael A, Aschner M, Bowman AB, Lafayette W (2021) Manganese acts upon insulin/IGF receptors to phosphorylate AKT and increase glucose uptake in Huntington’s disease cells. Mol Neurobiol 57:1570–1593. https://doi.org/10.1007/s12035-019-01824-1.Manganese
Aguirre V, White MF (2000) Dysregulation of IRS-proteins causes insulin resistance and diabetes. Current Opin Endocrinol Diabetes 7:1–7. https://doi.org/10.1097/00060793-200002000-00001
Gao L, Yuan P, Zhang Q, Fu Y, Hou Y, Wei Y, Zheng X, Feng W (2020) Taxifolin improves disorders of glucose metabolism and water-salt metabolism in kidney via PI3K/AKT signaling pathway in metabolic syndrome rats. Life Sci 263:118713. https://doi.org/10.1016/j.lfs.2020.118713
Petersen MC, Shulman GI (2018) Mechanisms of insulin action and insulin resistance. Physiol Rev 98:2133–2223. https://doi.org/10.1152/physrev.00063.2017
Samuel VT, Shulman GI (2012) Review mechanisms for insulin resistance: common threads and missing links. Cell 148:852–871. https://doi.org/10.1016/j.cell.2012.02.017
Miyake Y, Tanaka K, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y, Yamada T, Oeda T, Miki T, Kawamura N, Sakae N, Fukuyama H, Hirota Y, Nagai M (2010) Case-control study of risk of Parkinson’s disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J Neurol Sci 293:82–86. https://doi.org/10.1016/j.jns.2010.03.002
Song Q, Liu J, Dong L, Wang X, Zhang X (2021) Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed Pharmacother 140:111750. https://doi.org/10.1016/j.biopha.2021.111750
Domingueti CP, Dusse LMSA, Carvalho MDG, De Sousa LP, Gomes KB, Fernandes AP (2016) Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complicat 30:738–745. https://doi.org/10.1016/j.jdiacomp.2015.12.018
Horii N, Hasegawa N, Fujie S, Uchida M, Iemitsu M (2020) Resistance exercise-induced increase in muscle 5α-dihydrotestosterone contributes to the activation of muscle Akt/MTOR/P70S6K- and Akt/AS160/GLUT4-signaling pathways in type 2 diabetic rats. FASEB J 34:11047–11057. https://doi.org/10.1096/fj.201903223RR
Choi J, Kim KJ, Koh EJ, Lee BY (2018) Gelidium elegans extract ameliorates type 2 diabetes via regulation of MAPK and PI3K/Akt signaling. Nutrients. https://doi.org/10.3390/nu10010051
Archuleta TL, Lemieux AM, Saengsirisuwan V, Teachey MK, Lindborg KA, Kim JS, Henriksen EJ (2009) Oxidant stress-induced loss of IRS-1 and IRS-2 proteins in rat skeletal muscle: role of P38 MAPK. Free Radic Biol Med 47:1486–1493. https://doi.org/10.1016/j.freeradbiomed.2009.08.014
Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, Accili D (2000) Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. Journal of Clinical Investigation 105:199–205. https://doi.org/10.1172/JCI7917
Yang Z, Zhang L, Liu J, Lu F, Wang L, Chen Y, Li D (2019) Hypoglycemic effects of Esculeoside A are mediated via activation of AMPK and upregulation of IRS-1. BMC Complement Altern Med 19:1–9. https://doi.org/10.1186/s12906-019-2543-3
White MF (2002) IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. https://doi.org/10.1152/ajpendo.00514.2001
Zheng M, Wang P (2021) Role of insulin receptor substance - 1 modulating PI3K / Akt insulin signaling pathway in Alzheimer’s Disease. 3 Biotech 11:1–17. https://doi.org/10.1007/s13205-021-02738-3
Maiti P, Scott J, Sengupta D, Al-Gharaibeh A, Dunbar GL (2019) Curcumin and solid lipid curcumin particles induce autophagy, but inhibit mitophagy and the PI3K-Akt/MTOR pathway in cultured glioblastoma cells. Int J Mol Sci. https://doi.org/10.3390/ijms20020399
Ferreira LSS, Fernandes CS, Vieira MNN, De Felice FG (2018) Insulin resistance in Alzheimer’s disease. Front Neurosci 12:1–11. https://doi.org/10.3389/fnins.2018.00830
Chaudhuri J, Bains Y, Guha S, Kahn A, Hall D, Bose N, Gugliucci A, Kapahi P (2018) The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metab 28:337–352. https://doi.org/10.1016/j.cmet.2018.08.014
Salahuddin P, Rabbani G, Khan RH (2014) The role of advanced glycation end products in various types of neurodegenerative disease: a therapeutic approach. Cell Mol Biol Lett 19:407–437. https://doi.org/10.2478/s11658-014-0205-5
Li J, Liu D, Sun L, Lu Y, Zhang Z (2012) Advanced glycation end products and neurodegenerative diseases: mechanisms and perspective. J Neurol Sci 317:1–5. https://doi.org/10.1016/j.jns.2012.02.018
Abedini A, Derk J, Schmidt AM (2018) The Receptor for advanced glycation endproducts is a mediator of toxicity by IAPP and other proteotoxic aggregates: establishing and exploiting common ground for novel amyloidosis therapies. Protein Sci 27:1166–1180. https://doi.org/10.1002/pro.3425
Giridharan VV, Generoso JS, Collodel A, Dominguini D, Faller CJ, Tardin F, Bhatti GS, Petronilho F, Dal-Pizzol F, Barichello T (2021) Receptor for advanced glycation end products (RAGE) mediates cognitive impairment triggered by pneumococcal meningitis. Neurotherapeutics 18:640–653. https://doi.org/10.1007/s13311-020-00917-3
Cai W, He JC, Zhu L, Chen X, Striker GE, Vlassara H (2008) AGE-receptor-1 counteracts cellular oxidant stress induced by AGEs via negative regulation of P66shc-dependent FKHRL1 phosphorylation. Am J Physiol Cell Physiol. https://doi.org/10.1152/ajpcell.00350.2007
Hu P, Lai D, Lu P, Gao J, He H (2012) ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int J Mol Med 29:613–618. https://doi.org/10.3892/ijmm.2012.891
Yamamoto M, Guo DH, Hernandez CM, Stranahan AM (2019) Endothelial Adora2a activation promotes blood-brain barrier breakdown and cognitive impairment in mice with diet-induced insulin resistance. J Neurosci 39:4179–4192. https://doi.org/10.1523/JNEUROSCI.2506-18.2019
Sun Y, Ma C, Sun H, Wang H, Peng W, Zhou Z, Wang H, Pi C, Shi Y, He X (2020) Metabolism: a novel shared link between diabetes mellitus and Alzheimer’s disease. J Diabetes Res. https://doi.org/10.1155/2020/4981814
Akhtar A, Sah SP (2020) Insulin signaling pathway and related molecules: role in neurodegeneration and Alzheimer’s disease. Neurochemistry International 135:104707. https://doi.org/10.1016/j.neuint.2020.104707
Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R (2010) Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA 107:7036–7041. https://doi.org/10.1073/pnas.1000645107
Chen L, Wei Y, Wang X, He R (2010) Ribosylation rapidly induces α -synuclein to form highly cytotoxic molten globules of advanced glycation end products. PLoS One. https://doi.org/10.1371/journal.pone.0009052
Reyes ET, Perurena OH, Festoff BW, Jorgensen R, Moore WV (1984) Insulin resistance in amyotrophic lateral sclerosis. J Neurol Sci 63:317–324. https://doi.org/10.1016/0022-510X(84)90154-0
De Pablo-Fernandez E, Goldacre R, Pakpoor J, Noyce AJ, Warner TT (2018) Association between diabetes and subsequent Parkinson disease: a record-linkage cohort study. Neurology 91:e139–e142. https://doi.org/10.1212/WNL.0000000000005771
Delamarre A, Rigalleau V, Meissner WG (2020) Insulin resistance, diabetes and Parkinson’s disease: the match continues. Parkinsonism Relat Disord 80:199–200. https://doi.org/10.1016/j.parkreldis.2020.10.013
Kotagal V, Albin RL, Müller MLTM, Koeppe RA, Frey KA, Bohnen NI (2013) Diabetes is associated with postural instability and gait difficulty in Parkinson disease. Parkinsonism Relat Disord 19:522–526. https://doi.org/10.1016/j.parkreldis.2013.01.016
Brauer R, Wei L, Ma T, Athauda D, Girges C, Vijiaratnam N, Auld G, Whittlesea C, Wong I, Foltynie T (2020) Diabetes medications and risk of Parkinson’s disease: a cohort study of patients with diabetes. Brain 143:3067–3076. https://doi.org/10.1093/brain/awaa262
Schernhammer E, Hansen J, Rugbjerg K, Wermuth L, Ritz B (2011) Diabetes and the risk of developing Parkinson’s disease in Denmark. Diabetes Care 34:1102–1108. https://doi.org/10.2337/dc10-1333
Chen K, Xie K, Liu Z, Nakasone Y, Sakao K, Hossain A, Hou DX (2019) Preventive effects and mechanisms of garlic on dyslipidemia and gut microbiome dysbiosis. Nutrients. https://doi.org/10.3390/nu11061225
Gagnum V, Stene LC, Jenssen TG, Berteussen LM, Sandvik L, Joner G, Njølstad PR, Skrivarhaug T (2017) Causes of death in childhood-onset type 1 diabetes: long-term follow-up. Diabetic Med 34:56–63. https://doi.org/10.1111/dme.13114
Yin X, Xu Z, Zhang Z, Li L, Pan Q, Zheng F, Li H (2017) Association of PI3K/AKT/MTOR pathway genetic variants with type 2 diabetes mellitus in Chinese. Diabetes Res Clin Pract 128:127–135. https://doi.org/10.1016/j.diabres.2017.04.002
Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14:88–98. https://doi.org/10.1038/nrendo.2017.151
Li Y, Liu Y, Liang J, Wang T, Sun M, Zhang Z (2019) Gymnemic acid ameliorates hyperglycemia through PI3K/AKT- and AMPK-Mediated signaling pathways in type 2 diabetes mellitus rats. J Agric Food Chem 67:13051–13060. https://doi.org/10.1021/acs.jafc.9b04931
Walter-höliner AI, Barbarini DS, Lütschg J, Zanier U, Saely CH, Simma B (2017) High prevalence and incidence of diabetic peripheral neuropathy in children and adolescents with type 1 diabetes mellitus: results from a 5-year prospective cohort study. Pediatric Neurol. https://doi.org/10.1016/j.pediatrneurol.2017.11.017
Kallinikou D, Soldatou A, Tsentidis C, Louraki M, Kanaka-Gantenbein C, Kanavakis E, Karavanaki K (2019) Diabetic neuropathy in children and adolescents with type 1 diabetes mellitus: diagnosis, pathogenesis, and associated genetic markers. Diabetes/Metab Res Rev 35:1–14. https://doi.org/10.1002/dmrr.3178
Dong J, Li H, Bai Y, Wu C (2019) Muscone ameliorates diabetic peripheral neuropathy through activating AKT/MTOR signalling pathway. J Pharm Pharmacol 71:1706–1713. https://doi.org/10.1111/jphp.13157
Liu K, Yang Y, Zhou F, Xiao Y, Shi L (2020) Inhibition of PI3K/AKT/MTOR signaling pathway promotes autophagy and relieves hyperalgesia in diabetic rats. NeuroReport. https://doi.org/10.1097/WNR.0000000000001461
Biosa A, Outeiro TF, Bubacco L, Bisaglia M (2018) Diabetes mellitus as a risk factor for Parkinson’s disease: a molecular point of view. Mol Neurobiol 55:8754–8763. https://doi.org/10.1007/s12035-018-1025-9
Gao JR, Qin XJ, Fang ZH, Li-Shan, Han LP, Hui-Jian Guo MF, Jiang NN (2019) To explore the pathogenesis of vascular lesion of type 2 diabetes mellitus based on the PI3K/Akt signaling pathway. J Diabetes Res. https://doi.org/10.1155/2019/4650906
Hou Y, Wang K, Wan W, Cheng Y, Pu X, Ye X (2018) Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/MTOR pathway after stroke in rats. Genes Dis 5:245–255. https://doi.org/10.1016/j.gendis.2018.06.001
Du C, Wu M, Liu H, Ren Y, Du Y, Wu H, Wei J, Liu C, Yao F, Wang H, Zhu Y, Duan H, Shi Y (2016) Thioredoxin-interacting protein regulates lipid metabolism via Akt/MTOR pathway in diabetic kidney disease. Int J Biochem Cell Biol 79:1–13. https://doi.org/10.1016/j.biocel.2016.08.006
Ran Z, Zhang Y, Wen X, Ma J (2019) Curcumin inhibits high glucose-induced inflammatory injury in human retinal pigment epithelial cells through the ROS-PI3K/AKT/MTOR signaling pathway. Mol Med Reports 19:1024–1031. https://doi.org/10.3892/mmr.2018.9749
Akhtar A, Sah SP (2020) Neurochemistry international insulin signaling pathway and related molecules: role in neurodegeneration and Alzheimer’s disease. Neurochem Int 135:104707. https://doi.org/10.1016/j.neuint.2020.104707
Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, Mikolás E, Szijártó IA, Mérei Á, Halmai R, Mészáros LG, Sümegi B, Wittmann I (2011) Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in Type 2 diabetic patients. Br J Nutr 106:383–389. https://doi.org/10.1017/S0007114511000316
Chi H, Chang H, Sang T (2018) Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci. https://doi.org/10.3390/ijms19103082
Juranek JK, Daffu GK, Wojtkiewicz J, Lacomis D, Kofler J, Schmidt AM (2015) Receptor for advanced glycation end products and its inflammatory ligands are upregulated in amyotrophic lateral sclerosis. Front Cell Neurosci 9:1–12. https://doi.org/10.3389/fncel.2015.00485
Ray R, Juranek JK, Rai V (2016) RAGE axis in neuroinflammation, neurodegeneration and its emerging role in the pathogenesis of amyotrophic lateral sclerosis. Neurosci Biobehav Rev 62:48–55. https://doi.org/10.1016/j.neubiorev.2015.12.006
Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J (2014) The role of PI3K/AKT/MTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 26:2694–2701. https://doi.org/10.1016/j.cellsig.2014.08.019
Fakhri S, Iranpanah A, Mehdi M, Zachariah S (2021) Phytomedicine natural products attenuate PI3K / Akt / MTOR signaling pathway: a promising strategy in regulating neurodegeneration. Phytomedicine 91:153664. https://doi.org/10.1016/j.phymed.2021.153664
Soutar MPM, Kempthorne L, Miyakawa S, Annuario E, Melandri D, Harley J, O’Sullivan GA, Wray S, Hancock DC, Cookson MR, Downward J, Carlton M, Plun-Favreau H (2018) AKT signalling selectively regulates PINK1 mitophagy in SHSY5Y cells and human IPSC-derived neurons. Sci Reports 8:1–11. https://doi.org/10.1038/s41598-018-26949-6
Nguyen TT, Ta QTH, Nguyen TKO, Nguyen TTD, Van Giau V (2020) Type 3 diabetes and its role implications in Alzheimer’s disease. Int J Mol Sci 21:1–16. https://doi.org/10.3390/ijms21093165
Bendor J, Logan T, Edwards RH (2014) The function of α -synuclein. Neuron 79:1044–1066. https://doi.org/10.1016/j.neuron.2013.09.004
Bruyère J, Abada YS, Vitet H, Fontaine G, Deloulme JC, Cès A, Denarier E, Pernet-Gallay K, Andrieux A, Humbert S, Potier MC, Delatour B, Saudou F (2020) Presynaptic APP levels and synaptic homeostasis are regulated by Akt phosphorylation of huntingtin. elife 9:1–30. https://doi.org/10.7554/eLife.56371
Singh SS, Jois SD (2018) Homo- and heterodimerization of proteins in cell signaling: inhibition and drug design. Adv Protein Chem Struct Biol 111:1–59
Ghasemi R, Moosavi M, Zarifkar A, RastegarMaghsoudi KN (2015) The interplay of Akt and ERK in Aβ toxicity and insulin-mediated protection in primary hippocampal cell culture. J Mol Neurosci 57:325–334. https://doi.org/10.1007/s12031-015-0622-6
Kamynina AV, Esteras N, Koroev DO, Bobkova NV, Balasanyants SM, Simonyan RA, Avetisyan AV, Abramov AY, Volpina OM, Walker DG (2018) Synthetic fragments of receptor for advanced glycation end products bind beta-amyloid 1–40 and protect primary brain cells from beta-amyloid toxicity. Front Neurosci 12:1–9. https://doi.org/10.3389/fnins.2018.00681
Angeloni C, Zambonin L, Hrelia S (2014) Role of methylglyoxal in Alzheimer’s disease. Biomed Res Int 2014:238485
Zeng F, Liu Y, Huang W, Qing H, Kadowaki T, Kashiwazaki H, Ni J, Wu Z (2021) Receptor for advanced glycation end products up-regulation in cerebral endothelial cells mediates cerebrovascular-related amyloid β accumulation after porphyromonas gingivalis infection. J Neurochem 158:724–736. https://doi.org/10.1111/jnc.15096
Westwoo W, Beiser A, DeCarli C, Harris TB, Chen TC, He XM, Roubenoff R, Pikula A, Au R, Braverman LE, Wolf PA, Vasan RS, Seshadri S (2014) Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology 82:1613–1619. https://doi.org/10.1212/WNL.0000000000000382
Sedzikowska A, Szablewski L (2021) Insulin and insulin resistance in Alzheimer’s disease. Int J Mol Sci 22:9987
Su M, Naderi K, Samson N, Youssef I, Fülöp L, Bozso Z, Laroche S, Delatour B, Davis S (2019) Mechanisms associated with type 2 diabetes as a risk factor for Alzheimer-related pathology. Mol Neurobiol 56:5815–5834. https://doi.org/10.1007/s12035-019-1475-8
Lee H-K, Kwon B, Lemere CA, de la Monte S, Itamura K, Ha AY, Querfurth HW (2016) MTORC2 (Rictor) in Alzheimer’s disease and reversal of amyloid- β expression-induced insulin resistance and toxicity in rat primary cortical neurons. J Alzheimers Dis 56:1015–1036
Chong ZZ, Shang YC, Wang S, Maiese K (2012) A critical kinase cascade in neurological disorders: PI3K, Akt and MTOR. Future Neurol 7:733–748. https://doi.org/10.2217/fnl.12.72
Sun E, Motolani A, Campos L, Lu T (2022) The pivotal role of NF-KB in the pathogenesis and therapeutics of Alzheimer’s disease. Int J Mol Sci 23:8972. https://doi.org/10.3390/ijms23168972
Ali T, Kim MO (2015) Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3β pathway in the mouse hippocampus. J Pineal Res 59:47–59. https://doi.org/10.1111/jpi.12238
Wang A, Xiao C, Zheng J, Ye C, Dai Z, Wu Q, Liu J, Strappe P, Zhou Z (2020) Terpenoids of ganoderma lucidum reverse cognitive impairment through attenuating neurodegeneration via suppression of PI3K/AKT/MTOR expression in vivo model. J Funct Foods 73:104142. https://doi.org/10.1016/j.jff.2020.104142
Gasparotto J, Girardi CS, Somensi N, Ribeiro CT, Moreira JCF, Michels M, Sonai B, Rocha M, Steckert AV, Barichello T, Quevedo J, Dal-pizzol F, Gelain DP (2018) Cro receptor for advanced glycation end products mediates sepsis-triggered amyloid-  accumulation, tau phosphoryla- tion, and cognitive impairment. J Biol Chem 293:226–244. https://doi.org/10.1074/jbc.M117.786756
Athauda D, Foltynie T (2016) The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today 21:802–818. https://doi.org/10.1016/j.drudis.2016.01.013
Cheong JLY, de Pablo-Fernandez E, Foltynie T, Noyce AJ (2020) The association between type 2 diabetes mellitus and Parkinson’s disease. J Parkinson’s Dis 10:775–789. https://doi.org/10.3233/jpd-191900
Nakano N, Matsuda S, Ichimura M, Minami A, Ogino M, Murai T, Kitagishi Y (2017) PI3K/AKT signaling mediated by g protein-coupled receptors is involved in neurodegenerative Parkinson’s disease (review). Int J Mol Med 39:253–260. https://doi.org/10.3892/ijmm.2016.2833
Annekatrin K (2018) Alpha-synuclein glycation and the action of anti-diabetic agents in Parkinson’s disease. J Parkinsons Dis 8:33–43. https://doi.org/10.3233/JPD-171285
Natale G, Pompili E, Biagioni F, Paparelli S, Lenzi P, Fornai F (2013) Histochemical approaches to assess cell-to-cell transmission of misfolded proteins in neurodegenerative diseases. Eur J Histochem 57:31–35. https://doi.org/10.4081/ejh.2013.e5
Ashraghi MR, Pagano G, Polychronis S, Niccolini F, Politis M (2016) Parkinson’s disease, diabetes and cognitive impairment. Recent Pat Endocr Metab Immune Drug Discov 10:11–21. https://doi.org/10.2174/1872214810999160628105549
Wang SY, Wu SL, Chen TC, Chuang C. Sen (2020) Antidiabetic agents for treatment of Parkinson’s disease: a meta-analysis. Int J Environ Res Public Health 17:1–11. https://doi.org/10.3390/ijerph17134805
Becker C, Brobert GP, Johansson S, Jick SS, Meier CR (2008) Diabetes in patients with idiopathic Parkinson’s disease. Diabetes Care 31:1808–1812. https://doi.org/10.2337/dc08-0479
Hu MTM, Taylor-Robinson SD, Chaudhuri KR, Bell JD, Labbé C, Cunningham VJ, Koepp MJ, Hammers A, Morris RG, Turjanski N, Brooks DJ (2000) Cortical dysfunction in non-demented Parkinson’s disease patients. A combined 31P-MRS and 18FDG-PET study. Brain 123:340–352. https://doi.org/10.1093/brain/123.2.340
Shaikh S, Nicholson LFB (2008) Advanced glycation end products induce in vitro cross-linking of a -synuclein and accelerate the process of intracellular inclusion body formation. J Neurosci Res 2082:2071–2082. https://doi.org/10.1002/jnr.21644
Teismann P, Sathe K, Bierhaus A, Leng L, Martin HL, Bucala R, Weigle B, Nawroth PP, Schulz JB (2012) Receptor for advanced glycation endproducts (RAGE) deficiency protects against MPTP toxicity. NBA 33:2478–2490. https://doi.org/10.1016/j.neurobiolaging.2011.12.006
Sharma N, Pavani S, Kalivendi SV (2019) Free radical biology and medicine the deglycase activity of DJ-1 mitigates α -synuclein glycation and aggregation in dopaminergic cells : role of oxidative stress mediated downregulation of DJ-1 in Parkinson’s disease. Free Radic Biol Med 135:28–37. https://doi.org/10.1016/j.freeradbiomed.2019.02.014
Murata H, Sakaguchi M, Jin Y, Sakaguchi Y, Futami JI, Yamada H, Kataoka K, Huh NH (2011) A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: activation of AKT via MTORC2. J Biol Chem 286:7182–7189. https://doi.org/10.1074/jbc.M110.179390
Gugliandolo A, Pollastro F, Bramanti P, Mazzon E (2020) Cannabidiol exerts protective effects in an in vitro model of Parkinson’s disease activating AKT/MTOR pathway. Fitoterapia 143:104553. https://doi.org/10.1016/j.fitote.2020.104553
Bryan MR, Bowman AB (2017) Manganese and the insulin-IGF signaling network in huntington’s disease and other neurodegenerative disorders. Adv Neurobiol 18:113–142. https://doi.org/10.1007/978-3-319-60189-2_6
Ersoy Tunalı N (2021) Molecular mechanisms of polyglutamine pathology and lessons learned from Huntington’s disease. Neurodegener Dis. https://doi.org/10.5772/intechopen.93508
Colin E, Régulier E, Perrin V, Dürr A, Brice A, Aebischer P, Déglon N, Humbert S, Saudou F (2005) Akt is altered in an animal model of Huntington’s disease and in patients. Eur J Neurosci 21:1478–1488. https://doi.org/10.1111/j.1460-9568.2005.03985.x
Ehinger Y, Bruyère J, Panayotis N, Abada Y, Borloz E, Matagne V, Scaramuzzino C, Vitet H, Delatour B, Saidi L, Villard L, Saudou F, Roux J (2020) Huntingtin phosphorylation governs BDNF homeostasis and improves the phenotype of Mecp 2 knockout mice. EMBO Mol Med. https://doi.org/10.15252/emmm.201910889
Liévens JC, Iché M, Laval M, Faivre-Sarrailh C, Birman S (2008) AKT-sensitive or insensitive pathways of toxicity in glial cells and neurons in drosophila models of Huntington’s disease. Hum Mol Genet 17:882–894. https://doi.org/10.1093/hmg/ddm360
Bras IC, Konig A (2019) Glycation in Huntington’s disease : a possible modifier and target for intervention. J Huntingtons Dis 8:245–256. https://doi.org/10.3233/JHD-190366
Timothy Hansen CT (2019) Excess active P13K rescues Huntingtin-mediated neuronal cell death but has no effect on axonal transport defects. Apoptosis 176:341–358. https://doi.org/10.1007/s10495-019-01520-4.Excess
Abd-Elrahman KS, Ferguson SSG (2019) Modulation of MTOR and CREB pathways following MGluR5 blockade contribute to improved Huntington’s pathology in ZQ175 mice. Mol Brain 12:1–9. https://doi.org/10.1186/s13041-019-0456-1
Saberi S, Stauffer JE, Schulte DJ, Ravits J (2015) Neuropathology of amyotrophic lateral sclerosis and its variants. Neurol Clin 33:855–876. https://doi.org/10.1016/j.ncl.2015.07.012
Pradat P-F, Bruneteau G, Gordon PH, Dupuis L, Bonnefont-Rousselot D, Simon D, Salachas F, Corcia P, Frochot V, Lacorte J-M, Jardel C, Coussieu C, Le Forestier N, Lacomblez L, Loeffler J-P, Vincent VM (2010) Impaired glucose tolerance in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler 11(1–2):166–171
Kato S, Nakashima K, Horiuchi S, Nagai R, Cleveland DW, Liu J, Hirano A, Takikawa M, Kato M, Nakano I, Sakoda S, Asayama K, Ohama E (2001) Formation of advanced glycation end-product-modified superoxide dismutase-1 (SOD1) is one of the mechanisms responsible for inclusions common to familial amyotrophic lateral sclerosis patients with SOD1 gene mutation, and transgenic mice expressing human. Neuropathology. https://doi.org/10.1046/j.1440-1789.2001.00359.x
Juranek JK, Daffu GK, Geddis MS, Li H, Rosario R, Kaplan BJ, Kelly L, Schmidt AM (2016) Soluble RAGE treatment delays progression of amyotrophic lateral sclerosis in SOD1 mice. Front Cell Neurosci 10:1–9. https://doi.org/10.3389/fncel.2016.00117
Kikuchi S, Shinpo K, Ogata A, Tsuji S, Takeuchi M, Makita Z, Tashiro K (2002) Detection of Nε-(Carboxymethyl)Lysine (CML) and non-CML advanced glycation end-products in the anterior horn of amyotrophic lateral sclerosis spinal cord. Amyotroph Lateral Scler Other Motor Neuron Disord 3:63–68. https://doi.org/10.1080/146608202760196020
Kaufmann E, Boehm BO, Süssmuth SD, Kientsch-Engel R, Sperfeld A, Ludolph AC, Tumani H (2004) The advanced glycation end-product N ε-(Carboxymethyl)Lysine level is elevated in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Neurosci Lett 371:226–229. https://doi.org/10.1016/j.neulet.2004.08.071
Yin X, Ren M, Jiang H, Cui S, Wang S, Jiang H, Qi Y, Wang J, Wang X, Dong G, Leeds P, Chuang DM, Feng H (2015) Downregulated AEG-1 together with inhibited PI3K/Akt pathway is associated with reduced viability of motor neurons in an ALS model. Mol Cell Neurosci 68:303–313. https://doi.org/10.1016/j.mcn.2015.08.009
Tolosa L, Mir M, Asensio J, Olmos G (2008) Vascular endothelial growth factor protects spinal cord motoneurons against glutamate-induced excitotoxicity via phosphatidylinositol 3-kinase. J Neurochem 105:1080–1090. https://doi.org/10.1111/j.1471-4159.2007.05206.x
Funding
The authors declare that no funding and grants were accepted for the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed in manuscript design and study conception. Kanagavalli Ramasubbu collected the data and wrote the manuscript. Devi Rajeswari V contributed to the drafting of the manuscript. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramasubbu, K., Devi Rajeswari, V. Impairment of insulin signaling pathway PI3K/Akt/mTOR and insulin resistance induced AGEs on diabetes mellitus and neurodegenerative diseases: a perspective review. Mol Cell Biochem 478, 1307–1324 (2023). https://doi.org/10.1007/s11010-022-04587-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04587-x