Abstract
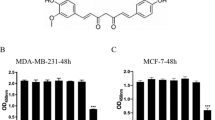
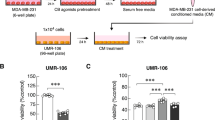
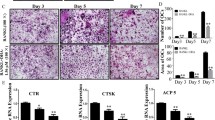
UBS109 is a curcumin analog that possesses antitumor properties has been shown to stimulate osteoblastogenesis and suppress osteoclastogenesis in vitro. This study was undertaken to determine whether UBS109 might alleviate the inhibitory activity of breast cancer cells on osteoblastic mineralization and stimulatory effects on osteoclastogenesis. Mouse bone marrow cells were cocultured with breast cancer MDA-MB-231 bone metastatic cells in vitro. UBS109 stimulated osteoblastic mineralization and suppressed adipogenesis and osteoclastogenesis in bone marrow culture. Coculture with MDA-MB-231 cells suppressed osteoblastic mineralization and enhanced osteoclastogenesis in bone marrow culture. Effects that were reserved by UBS109 (50–200 nM). Mineralization in preosteoblastic MC3T3-E1 cells was suppressed by coculture with MDA-MB-231 cells, while MDA-MB-231 cells did not have effects on osteoclastogenesis of RAW267.4 cells in vitro. UBS109 (500 nM) revealed toxic effects on MDA-MB-231 bone metastatic cells. This study demonstrates that UBS109, which is an antitumor agent, reveals restorative effects on bone marrow cell differentiation disordered by coculture with breast cancer MDA-MB-231 bone metastatic cells in vitro. This in vitro model may be a useful tool to evaluate the mechanism of breast cancer cell bone metastasis.








Similar content being viewed by others
References
Raggatt LJ, Partridge NC (2010) Cellular and molecular mechanisms of bone modeling. J Biol Chem 285:25103–25108
Weitzmann MN, Pacifici R (2006) Estrogen deficiency and bone loss: an inflammatory tale. J Clin Investig 116:1186–1194
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
Boyce BF, Yoneda T, Guise TA (1999) Factors regulating the growth of metastasis cancer in bone. Endocr Relat Cancer 6:333–347
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2:584–593
Roodman CD (2004) Mechanism of bone metastasis. N Engl J Med 350:1655–1664
Akhtari M, Mansuri J, Newman KA, Guise TM, Seth P (2008) Biology of breast cancer bone metastasis. Cancer Biol Ther 7:3–9
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27:165–176
Chen Y-C, Sosnoski DM, Mastro AM (2010) Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Res 12:215
Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, McCauley L, Shi S, Chen S, Wang C-Y (2007) NF-κB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med 13:62–69
Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Enwert R, Branstetter D, Dougall WC (2010) RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 468:103–107
Zaidi M, Blair HC, Moonga BS, Abe E, Huang CL-H (2003) Osteoclastogenesis, bone resorption, and osteoblast-based therapeutics. J Bone Miner Res 18:599–609
Weilbaecher KN, Guise TA, McCauley LK (2011) Cancer to bone: a fatal attraction. Nat Rev Cancer 11:411–425
Sun A, Lu YJ, Hu H, Shoji M, Liotta DC, Snyder JP (2009) Curcumin analog cytotoxicity against breast cancer cells: exploitation of a redox-dependent mechanism. Bioorg Med Chem Lett 19:6627–6631
Zhu S, Moore TW, Lin X, Morii N, Mancini A, Howard RB, Culver D, Arrendale RF, Reddy P, Evers TJ, Zhang H, Sica G, Chen ZG, Sun A, Fu H, Khuri FR, Shin DM, Snyder JP, Shoji M (2012) Synthetic curcumin analog EF31 inhibits the growth of head and neck squamous cell carcinoma xenografts. Integr Biol (Camb) 4:633–640
Nagaraju GP, Zhu S, Wen J, Farris AB, Adsay VN, Diaz R, Snyder JP, Shoji M, El-Rayes BF (2013) Novel synthetic curcumin analogues EF31 and UBS109 are potent DNA hypomethylating agents in pancreatic cancer. Cancer Lett 341:195–203
Yamaguchi M, Moore TW, Sun A, Snyder JP, Shoji M (2012) Novel curcumin analogue UBS109 potently stimulates osteoblastogenesis and suppresses osteoclastogenesis through Smad activation and NF-κB Inhibition. Integr Biol (Camb) 4:905–913
Yamaguchi M, Zhu S, Zhang S, Wu D, Moore TM, Snyder JP, Shoji M (2014) Curcumin analogue UBS109 prevents bone loss in breast cancer bone metastasis mouse model: involvement in osteoblastogenesis and osteoclastogenesis. Cell Tissue Res 357:245–252
Yamaguchi M, Weitzmann MN, Baile CA, Murata T (2012) Exogenous regucalcin suppresses osteoblastogenesis and stimulates adipogenesis in mouse bone marrow culture. Integr Biol (Camb) 4:1215–1222
Minkin C (1982) Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker osteoclast function. Calcif Tissue Int 34:285–290
Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R (2001) A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res 16:1486–1495
Li Y, Li A, Strait K, Zhang H, Nanes MS, Weitzmann MN (2007) Endogenous TNFalpha lowers maximum peak bone mass and inhibits osteoblastic Smad activation, through NF-kappaB. J Bone Miner Res 22:646–655
Yamaguchi M, Weitzmann MN, Murata T (2012) Exogenous regucalcin stimulates osteoclastogenesis and suppresses osteoblastogenesis through NF-kB activation. Mol Cell Biochem 359:193–203
Muruganandan S, Roman AA, Sinal CJ (2009) Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci 66:236–253
Prusty D, Park BH, Davis KE, Farmer SR (2002) Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPAR gamma) and C/EBP alpha gene expression during the differentiation of 3T3-L1 preadipocytes. J Biol Chem 277:46226–46232
Wu L, Cai X, Dong H, Jing W, Huang Y, Yang X, Wu Y, Lin Y (2010) Serum regulates adipogenesis of mesenchymal stem cells via MEK/ERK-dependent PPARgamma expression and phosphorylation. J Cell Mol Med 14:922–932
Laudes M (2011) Role of WNT signaling in the determination of human mesenchymal stem cells into preadipocytes. J Mol Endocrinol 46:R65–R72
Acknowledgments
Dr. Toshiyuki Yoneda (The University of Texas, San Antonio, USA) kindly provided breast cancer MDA-MB-231 bone metastatic cells.
Conflict of interest
All authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamaguchi, M., Zhu, S., Weitzmann, M.N. et al. Curcumin analog UBS109 prevents bone marrow osteoblastogenesis and osteoclastogenesis disordered by coculture with breast cancer MDA-MB-231 bone metastatic cells in vitro. Mol Cell Biochem 401, 1–10 (2015). https://doi.org/10.1007/s11010-014-2286-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2286-x