Abstract
Context
Habitat fragmentation is a leading threat to biodiversity, yet the impacts of fragmentation on most taxa, let alone interactions among those taxa, remain largely unknown.
Objectives
We studied how three consequences of fragmentation—reduced patch connectivity, altered patch shape, and edge proximity—impact plant-dwelling mite communities and mite-plant-fungus interactions within a large-scale habitat fragmentation experiment.
Methods
We sampled mite communities from the leaves of Quercus nigra (a plant species that has foliar domatia which harbor fungivorous and predacious mites) near and far from edge within fragments of varying edge-to-area ratio (shape) and connectivity via corridors. We also performed a mite-exclusion experiment across these fragmentation treatments to test the effects of mite presence and fungal hyphal abundance on leaf surfaces.
Results
Habitat edges influenced the abundance and richness of leaf-dwelling mites; plants closer to the edge had higher mite abundance and species richness. Likewise, hyphal counts were higher on leaves near patch edges. Despite both mite and fungal abundance being higher at patch edges, leaf hyphal counts were not impacted by mite abundance on those leaves. Neither patch shape nor connectivity influenced mite abundance, mite species richness, or the influence of mites on leaf surface fungal abundance.
Conclusion
Our results suggest that mites and foliar fungi may be independently affected by edge-structured environmental gradients, like temperature, rather than trophic effects. We demonstrate that large-scale habitat fragmentation and particularly edge effects can have impacts on multiple levels of microscopic communities, even in the absence of cascading trophic effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat fragmentation is extensive across the globe (Haddad et al. 2015), yet how fragmentation influences biodiversity remains a point of active inquiry (Fletcher et al. 2018; Fahrig et al. 2019). Fragmentation may affect populations and communities through mechanisms such as reduced landscape connectivity, altered shape of habitat patches, and creation of edges, with experiments being a critical tool for disentangling these effects (Haddad et al. 2015; Fletcher et al. 2018). Fragmentation and its effects can also alter the outcomes of trophic and mutualistic interactions such as pollination, seed predation, seed dispersal, and herbivory, again through a variety of landscape alterations (e.g., Aguilar et al. 2006, Magrach et al. 2014, Levey et al. 2016). Yet, rarely have studies simultaneously considered how fragmentation effects influence the biodiversity of groups while also investigating impacts on their related trophic interactions. Studies that consider changes in both biodiversity and the outcomes of trophic interactions have the potential to provide novel insights into fragmentation effects. For example, habitat fragmentation and its effects could change the abundance or community makeup of a particular organismal group (e.g., herbivores or frugivorous birds) in ways that may or may not translate into modified trophic interactions (e.g., Evans et al. 2012, Mueller et al. 2014). Ultimately, a holistic understanding of habitat fragmentation will require disentangling direct effects of fragmentation on species from indirect effects resulting from modified species interactions.
Here, we investigate the impacts of habitat fragmentation on the biodiversity and trophic interactions of an ecologically important, but largely understudied, community: foliar mites that are associated with domatia. Domatia are small structures on the undersides of plant leaves that provide housing for predacious and fungivorous mites, thereby mediating a plant-mite defense mutualism (O’Dowd and Willson 1997; Romero and Benson 2005). By consuming deleterious fungi (Norton et al. 2000) and small herbivorous arthropods (Grostal and O’Dowd 1994; Agrawal and Karban 1997), mites living in domatia act as bodyguards for plants, reducing plant damage and increasing plant fitness (Walter 1996). In turn, domatia provide protection for mites against abiotic (e.g., desiccation, UV radiation) and biotic (e.g., predation) stressors (Grostal and O’Dowd 1994; Norton et al. 2001; Onzo et al. 2010). Mite domatia are a constitutive trait of the plant (they are present regardless of mite presence), and the number of domatia on a leaf typically positively correlates with mite abundance and species richness (Walter and O’Dowd 1992; Grostal and O’Dowd 1994). Mite domatia are both geographically and evolutionarily widespread across angiosperms, yet remain underexplored relative to other plant-arthropod mutualisms (Brouwer and Clifford 1990; O’Dowd and Pemberton 1994; Pearse et al. 2020). Additionally, the community composition and trophic function of domatia-dwelling mites can vary across environmental contexts (O’Dowd and Willson 1989; Pemberton and Turner 1989; Walter 1996), and it remains unclear how either is affected by fragmentation, or how effects could impact trophic outcomes of the plant-mite-fungal interactions.
Fragmentation may affect domatia-dwelling mite communities, leaf fungi, and their trophic interactions in several ways. Fragmentation—the conversion of contiguous habitat into smaller parcels following habitat destruction—can influence ecological systems across spatial scales, from localities to landscapes (Didham et al. 2012). A key research need is to resolve the mechanisms for these changes, which we refer to as fragmentation effects (Didham et al. 2012). In the context of this species interaction, fragmentation effects could directly alter mite and/or fungal communities by reducing habitat connectivity at the landscape scale, which can affect the ecological context, and thus the communities and interactions, of both the host plants of these microorganisms as well as the microorganisms themselves. Patch isolation has led to local extinctions and loss of biodiversity in other groups due to reduced gene flow between patches, loss of rescue effects, and altered species interactions (Didham et al. 2012; Haddad et al. 2015; Damschen et al. 2019). The negative outcomes of reduced connectivity have been documented in several macroarthropod species (Kruess and Tscharntke 1994; Zabel and Tscharntke 1998; Gibbs and Stanton 2001), as have effects of restoring connectivity through the creation or maintenance of corridors between isolated patches (Collinge 2000; Haddad et al. 2003; Öckinger et al. 2010). However, work on microarthropods is rare compared to larger organisms, and we still know relatively little about the impacts of habitat connectivity on microarthropod communities, let alone their trophic consequences. Although they were conducted on small scales (moss-island ecosystems only centimeters in size), the few experimental studies of the effects of fragmentation performed on microarthropods to date have pointed to reduced habitat connectivity as a cause of population decline and species loss (Gilbert et al. 1998; Gonzalez et al. 1998). While previous findings suggest that fragmentation effects and connectivity on the scale of hectares can have strong impacts on larger arthropods, such impacts have yet to be measured for microarthropods, and specifically in mite communities. Habitat connectivity could also directly impact fungal abundance on plant leaves, and previous work focused on leaf fungi has shown that connectivity can increase the incidence of fungal plant pathogens in fragmented habitats (Thrall et al. 2003; Laine and Hanski 2006).
Aside from direct effects, connectivity could also alter mite and fungal communities through trophic interactions, either indirectly impacting one trophic level via another (e.g., top-down or bottom-up effects), or by impacting the direction/magnitude of the trophic interaction itself. For instance, if reduced connectivity decreases mite abundance, leaf fungal abundance may increase in more isolated patches through a trophic interaction between mites and fungi. Connectivity could also impact the interaction itself: Huffaker (1958) found that increased connectivity was a key component of predator-prey coexistence among predacious and herbivorous mites, and experiments with larger arthropods have demonstrated that decreased patch connectivity disrupts trophic interactions (Martinson and Fagan 2014), and negatively affects insects at higher trophic levels more so than it affects the lower trophic levels they feed on (Brückmann et al. 2011). However, little work has investigated the impacts of connectivity and isolation on the trophic interactions of microarthropods at larger spatial scales over which habitat fragmentation and connectivity conservation typically take place. Ultimately, experimental manipulations are needed to disentangle trophic impacts from the direct effects of changes to fragment connectivity on lower trophic levels.
Aside from habitat connectivity effects, the impacts of fragmentation could also impact domatia-dwelling mite and fungal communities and their trophic interactions via the creation of edges. Edges are likely to impact mites and fungi by changing the microclimate within patches, manifesting as effects of edge proximity (i.e., distance to edge; Tuff et al. 2016). For example, by changing microclimate conditions, the presence of edges may alter plant-dwelling mite and fungal communities by altering desiccation risk, as both mites and fungi are sensitive to factors such as temperature and humidity (Walter and Proctor 2013, Ghazy and Suzuki 2014, Sullivan et al. 2011). In this system, patch edges are cooler and shadier than patch centers (Johnson and Haddad 2011), meaning that decreased desiccation risk in these regions of the patches could result in higher abundances of mites and fungi. The effects of edge proximity can additionally manifest as patch-level effects by changing the amount of a patch’s edge relative to its area, which in turn may affect populations and trophic interactions (Evans et al. 2012). For example, predatory mite communities in high-edge patches may experience different levels of food availability (in the form of other microarthropods or fungi) and altered abiotic climate factors compared to mite communities in patches of the same area but with less edge. Here, we refer to effects caused by changes in the edge-to-area ratio of a patch as effects of patch shape to distinguish these impacts from those caused by edge proximity.
Edge effects could also affect the trophic impacts of mites on leaf fungi. For instance, if microclimate conditions are more favorable for mites at patch edges, leaf fungal abundance may be indirectly driven by edge effects on mites through a trophic interaction between mites and fungi. Alternatively, edges could have impacts on leaf fungal abundance directly through means other than how they affect mites and the trophic interaction with fungi. For example, edges might directly alter leaf fungal abundance by producing more favorable microclimate conditions (decreased temperature, higher moisture, shade) for growth near edges (Sullivan et al. 2011). Both direct and indirect mechanisms may also be occurring simultaneously, and experiments are needed to understand their relative impacts on shaping overall patterns of leaf fungal abundance.
To better understand how habitat fragmentation influences biodiversity and trophic interactions, we conducted an experimental field study to disentangle the impacts of multiple habitat fragmentation effects on domatia-dwelling mites and mite-fungi interactions on water oak (Quercus nigra L.). We conducted a large-scale, replicated field experiment to isolate and resolve the effects of three consequences of habitat fragmentation for domatia-dwelling mites and leaf fungi on Q. nigra leaves: connectivity, edge proximity, and patch shape. To disentangle direct and indirect effects of fragmentation on mite-fungi interactions, we crossed this field experiment with a plant-level manipulation of the presence/absence of mites by experimentally blocking domatia, which near-entirely eliminates mite presence on plant leaves. We asked the following questions:
-
(1)
Do the effects of habitat fragmentation, specifically patch connectivity, patch shape, or proximity to the patch edge, impact the abundance and diversity of mites living on Q. nigra?
-
(2)
Do the effects of habitat fragmentation impact the outcome of trophic interactions between domatia-dwelling mites and leaf fungi on Q. nigra? In particular, is fungal abundance on leaves impacted by changes in patch connectivity, patch shape, or proximity to the patch edge, and if so, are these impacts direct or the result of an altered mite-fungi interaction across landscapes?
Methods
Study site
We worked within a long-running fragmentation experiment at the Savannah River Site (SRS), a National Environmental Research Park located near New Ellenton, South Carolina. The experiment consists of seven replicated experimental landscapes (blocks), each of which is 50 ha in size and includes five ~ 1.4 ha longleaf pine savanna patches surrounded by longleaf (Pinus palustris Mill.) and/or loblolly (Pinus taeda L.) pine plantation with limited herbaceous ground cover (Fig. 1A). Savanna patches were originally created by clearing mature pine plantation forests and managing the clearings with prescribed fire every 2–3 years to maintain open patch structure, as is typical for longleaf pine savanna system (Jose et al. 2006). Six of the seven blocks had been burned in the winter of 2017–2018 (the winter prior to our work), with the remaining block burned during the previous winter of 2016–2017. This design results in experimental patches of open savanna habitat surrounded by a forested matrix.
Diagram of the experimental design, showing (A) the patch arrangement within one experimental block (n = 7 total), and (B) center versus edge zones within an example patch. Comparisons of connectivity and patch shape hold patch area constant. The darker shaded regions represent the edge and center areas in which we selected oaks for our mite community sampling. For the fungal trophic interaction sampling, paired control and treatment oaks were selected to be as close to each other as was possible, within each edge distance zone
The design of the experiment isolates the effects of patch shape and connectivity while holding constant the area of individual patches and the total habitat area within each block. Each block includes a 100 × 100 m center patch with four peripheral patches each 150 m away from the center patch (Fig. 1A). A corridor (25 m wide and 150 m long) connects the center patch and one of the peripheral patches, and is included in the area of this “connected patch”. The unconnected patches are “rectangular” or “winged,” with equal areas (~ 1.4 ha, with the extra 0.4 ha area being the wings or back of the rectangle patch). Each experimental block has at least one rectangular and one winged patch and the remaining patch in each block was randomly assigned to be either a rectangular patch or a winged patch. Effects of connectivity can be distinguished through comparisons of connected and winged patches (which differ only in their connectivity), while patch shape is distinguished through comparing winged and rectangle patches (which differ only in their edge-to-area ratio).
Focal plant species
Quercus nigra (Fagaceae) is a deciduous oak tree species native to North America. This species is widespread within the experimental landscapes and has conspicuous mite domatia which take the form of clusters of trichomes located at the junctions of major veins on the lower leaf surface. While this is the first study to examine the mite communities associated with the domatia of Q. nigra, studies in other species (including oaks) have demonstrated that domatia are occupied by a group of largely fungivorous, but sometimes also predacious, mites from families such as Tydeidae and Phytoseiidae, which are considered plant mutualists (O’Dowd and Willson 1997). Due to recent prescribed fire, our sampled oaks were resprouting and relatively short (40–220 cm in height).
Do the effects of habitat fragmentation impact the abundance and diversity of mites living on Q. nigra leaves?
Experimental design
To test for fragmentation effects on mite communities, we sampled mites on leaves of Q. nigra plants from across all treatments in the field experiment. We sampled four Q. nigra individuals in the peripheral patches of each of the seven replicate blocks (Fig. 1A) in late June through early August of 2018 (n = 110 oaks in 28 patches, due to one patch that lacked the requisite number of oaks). We sampled two oaks in each of two zones within each patch (Fig. 1B): the edge (within 12.5 m of the patch edge) and the center (the patch area greater than 37.5 m from the patch edge). Within the two zones, we located oaks that were healthy and similar in size. After selecting trees, we haphazardly sampled 10 fully-expanded, undamaged leaves from each oak, and immediately placed these leaves in plastic bags containing moistened paper-towels for transport on ice from the field to the laboratory. Within the plastic bags, leaves did not come in contact with each other.
Within 48 h of collection, we examined the undersides of all leaves with a dissection microscope, counting the number of domatia per leaf and the total number of mites per leaf. Additionally, we sorted mites into morphospecies; representatives of which were stored in 95% ethanol and stored at −20 °C for later DNA extraction and identification. The methods for mite DNA extraction and barcoding can be found in the supplement (Appendix S1).
Statistical analysis
We used generalized linear mixed models (GLMM) to test relationships between habitat fragmentation and our response variables of interest. All analyses were performed in R, version 4.1.1 (R Core Team 2021).
To determine the overall impacts of habitat fragmentation on mite communities, we ran GLMMs with fixed effects of edge distance, patch type (connected, winged, or rectangle), number of domatia, and an edge distance by patch type interaction as predictor variables. We ran separate models with either mite abundance or richness as response variables. For our species richness response variable, we dropped the mites we classified as nymphs from the analysis, unless a nymph was the only morphospecies found on the leaf. For each model, we included a nested random effect of focal plant, nested in patch, nested in block to account for our experimental design. We used a negative binomial for our error distribution and ran these models through the glmmTMB package, version 1.0.1 (Brooks et al. 2017).
To test whether impacts of fragmentation on mite communities are mediated by changes in the number of domatia on leaves, we used GLMMs and a structural equation model (SEM). Microclimatic factors that distinguish patch edges from patch centers may also result in morphological differences in our plant species, and this potential effect of edge distance would function similarly regardless of patch type. Therefore, we considered the relationship between the number of domatia per leaf and distance from the edge of the patch using a GLMM with the same random effect structure as above, but with a Poisson error distribution, and implemented through the lme4 package, version 1.1–23 (Bates et al. 2015). Given the results of our GLMMs, we also constructed an SEM, in order to disentangle the direct effect of domatia on mites from the indirect effect of edge as mediated through the number of domatia (Fig. 2A). The structural equation model was built using the piecewiseSEM package, version 2.1.0 (Lefcheck 2016), with model statements written in the lme4 package. As explained above, we also included the number of domatia as an explanatory variable in our models examining mite abundance and richness. This SEM used data from 1100 individual leaves.
Do the effects of habitat fragmentation impact the outcome of trophic interactions between domatia-dwelling mites and leaf fungi on Q. nigra?
Experimental design
To investigate whether fragmentation effects altered plant-mite-fungus interactions, we applied a mite-exclusion treatment to two pairs of Q. nigra individuals, resulting in one control and one treatment oak at both the edge and center of the patches (n = 109 oak individuals among 28 patches, due to one mortality event and one patch that lacked the requisite number of oaks). We selected focal Q. nigra individuals for this experiment using the same edge and center zones and strategy as the mite diversity survey (Fig. 1B), although different individual plants were used. We excluded mites by blocking domatia with pruning tar, a standard manipulative technique for nearly eliminating domatia-dwelling mites on plant leaves (Norton et al. 2000, 2001; Monks et al. 2007). We applied pruning tar (TreeKote Wound Dressing, Walter E. Clark & Son, Inc., Orange, CT) to all of the domatia on the underside of every leaf of two treated trees in each patch, randomly assigned to the mite-exclusion treatment. For the remaining two Q. nigra trees in each patch, we conducted a control treatment to account for the presence of pruning tar (and any potential effects of the tar itself), by applying approximately the same number of tar spots that would have been applied to the domatia, but elsewhere on the lower leaf surface, such that the domatia were still available for mite colonization and use. Before applying the tar treatment, we pruned all focal Q. nigra individuals to a consistent size. We maintained the treatment on all leaves for six weeks, following initial tar application. Any new leaves that were produced during this time were removed, in order to maintain the treatment across the whole plant.
After the six-week treatment period, we haphazardly sampled three fully-expanded, undamaged leaves from each oak. We immediately placed these leaves in plastic bags containing moistened paper-towels for transport on ice from the field to the laboratory. All leaves were processed within 24 h of collection. For each leaf, we examined the leaf undersides under a dissection microscope, counting all domatia and the number of mites. To quantify fungal abundance on the leaf surface, we used established fungal peel methods (Harris 2000; Monks et al. 2007). In brief, following the counting of mites and domatia, we pressed the sticky side of a 19 mm wide piece of Matte Finish tape to the lower leaf surface (Skilcraft, Alexandria, Virginia, USA). We then placed the tape on a slide and dyed the fungal hyphae on the slide mount using a solution of 0.5% (w/v) trypan blue in lactoglycerol (1:1:1 lactic acid, glycerol and water filtered at 0.45 μm). We counted the number of times hyphal threads crossed a transect from a standardized area of tape (that being the whole width of the tape, from one side of the slide mount to the other).
Statistical analyses
To confirm that the mite-exclusion treatment effectively reduced or excluded mites on leaves, we ran a GLMM with the number of mites on the leaves as the response and the experimental treatment as the predictor, with a nested random effect of focal plant, nested within patch, nested within block to account for our experimental design. We used a negative binomial error distribution and implemented the model through the glmmTMB package, version 1.0.1.
To evaluate whether fungal abundance on leaves is impacted by changes in patch connectivity, patch shape, or proximity to the patch edge, and if so, whether these impacts are mediated by the presence or absence of mites, or the result of an altered mite-fungi interaction across landscapes, we ran a GLMM with hyphal count as our response variable. Our fixed effects were treatment, edge proximity, patch type, a treatment by edge proximity interaction term, and a treatment by patch type interaction term. Treatment in this model refers to the mite-exclusion treatment, which did effectively control mite abundance. We chose to include all mite morphotaxa for which we had data in this analysis. All of our observed families contain at least some fungivorous taxa and because these species are poorly understood and because, trophically, predators should be rare, we felt this was the more conservative approach for data analysis in this system. Our random effect followed the same nested structure as the mite community analyses (focal tree within patch within block). We used a negative binomial distribution to account for overdispersion in our hyphal count data. We implemented this model through the glmmTMB package, version 1.0.1 (Brooks et al. 2017).
To distinguish the direct effects of our habitat variables (patch shape, connectivity, distance from edge) on fungal abundance from the indirect effects of these variables, as mediated by mites or domatia, we constructed another SEM (Fig. 2B). As above, we used the piecewiseSEM package, version 2.1.0 (Lefcheck 2016), with model statements written in the lme4 package. For this analysis, we only included control leaves on which mites had not been excluded so that our results would not be confounded by domatia blockage. This SEM used data from 165 leaves.
Results
Do the effects of habitat fragmentation impact the abundance and species richness of mites living on Q. nigra leaves?
We counted 438 mites representing 15 different morphospecies on Q. nigra leaves. The most abundant morphospecies were from the families Tarsonemidae, Phytoseiidae, Tydeidae, and Eupodidae (Table S1, Walter and Proctor 2013). Of these common morphospecies, we were able to assign 36.5% of individuals to mite families that exhibit either fungivorous or omnivorous diets and 17.2% to families that exhibit primarily predatory diets (Table S1). Genbank accession numbers for individuals of barcoded morphospecies can be found in Table S1.
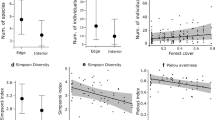
Edges, a common consequence of habitat fragmentation, affected mite communities by changing mite abundance and richness. We found a main effect of edge proximity on total mite abundance across all patch types (Table S2, Fig. 3A), where there were, on average 82% more mites on oak leaves at edges compared to patch centers (0.51 ± 0.046 mites/ leaf at edges, 0.28 ± 0.030 mites/leaf at patch centers, mean ± standard error). We found the same pattern for mite species richness, where there were, on average twice as many mite species on oak leaves at edges compared to patch centers (0.33 ± 0.023 species/ leaf at edges, 0.22 ± 0.020 species/leaf at patch centers, Fig. 3B). We did not find evidence for a main effect of connectivity (connected vs. winged patches), or patch shape (winged vs. rectangle patches), or interactions between the type of patch (connected, rectangle, winged) and edge proximity on mite species abundance or richness (Table S2, Fig. 3).
Estimated effects of connectivity (wing - connected), edge-to-area ratio (wing - rectangle), and distance from the edge (edge - center) on A mite abundance and B mite species richness from our statistical models. Significant differences are noted with *P < 0.05. Error bars are standard error of the mean
We next asked whether fragmentation effects were mediated by changes to the number of domatia on leaves, or whether these effects impacted mite communities directly, independent of any changes to leaf domatia abundance. Through our structural equation model (Fig. 2A), we found that edges affect mite abundance, both directly and indirectly through modifying the number of domatia. Distance from the edge is negatively associated with mite abundance (r=−0.22, p = 0.04) and with the number of domatia (r=−0.03, p = 0.04). There were on average 13.3% more domatia on leaves of oaks at the edges of the patches. The number of domatia is positively related to mite abundance (r = 0.26, p = 0.0006, Pearson’s r of 0.11). Neither patch connectivity nor patch shape was significantly related to mite abundance (r = 0.16, p = 0.25 and r = 0.10, p = 0.45, respectively). The SEM fit the data well (Fisher’s C = 1.80, p = 0.77).
Because leaf size could be influenced by edge proximity in our system and could in turn influence the number of domatia and mites on a given leaf, we additionally tested for the presence of these correlations on a subset of our experimental leaves. Leaf area was positively correlated with edge distance (p = 0.018) and there was a trend for leaf area to correlate positively with domatia number (p = 0.065). These patterns are opposite those we would expect if leaf area drove our findings of higher mite richness/abundance near edge and, thus, do not explain our results.
Do the effects of habitat fragmentation impact the outcome of trophic interactions between domatia-dwelling mites and leaf fungi on Q. nigra?
We asked whether fungal abundance on leaves was impacted by patch connectivity, patch shape, or proximity to the patch edge, and if so, whether these impacts are mediated by changes in mite communities. There were 5.6% more fungal hyphae present on leaves at patch edges than in patch centers (p = 0.02), as calculated from estimated marginal means of the model (Fig. 4). However, there were no effects of connectivity (p > 0.05) or patch shape (p > 0.05) on fungal abundance (Fig. 4).
Estimated effects of connectivity, patch shape, edge proximity, and mite-exclusion via tar treatment on fungal hyphae abundance on Q. nigra leaf surfaces from our statistical models. Only the relationship between fungal abundance and edge proximity is significant (p = 0.02); the remaining comparisons are not (p > 0.05)
There was no evidence that the effects of habitat fragmentation impacted fungal abundance by changing mite-fungal trophic interactions. Blocking domatia decreased mite abundance (p = 0.004; with 107% more mites on control leaves than treatment leaves). However, there was no significant effect of the mite-exclusion treatment on fungal abundance (p = 0.13, Fig. 4). Additionally, there was no significant relationship between mite abundance and fungal abundance (Fig. S1), despite the fact that of the mites to which we were able to assign a feeding guild, 80% of mite individuals were fungivorous or omnivorous. The effect of the interaction of domatia blocking and distance from the patch edge on fungal abundance was nonsignificant (p = 0.16), as was the interaction of treatment with the type of patch (p = 0.96).
We additionally asked whether our landscape variables impacted fungal hyphae abundance directly, or if this relationship was mediated by the number of domatia per leaf or the abundance of mites per leaf, using an SEM on control leaves (those allowing mite access) from our experiment (Fig. 2B). We found that only edge proximity had a significant direct impact on fungal abundance (r = −0.006; p = 0.029). We did again observe a significant positive effect of domatia on mite abundance (r = 0.53; p = 0.0267). However, in contrast to our first SEM (Fig. 2A), we did not observe an impact of edge proximity on domatia nor on mites. This may be explained by the smaller sample size available for this SEM (n = 165 leaves). This SEM fit the data well (Fisher’s C = 1.19, p = 0.55).
Discussion
The effects of habitat fragmentation influenced mite communities on Q. nigra leaves through the creation of edges. Mite abundance and richness were greater near patch edges; fungal abundance was also higher near patch edges. However, these landscape variables did not shift trophic interactions between mites and leaf fungus. Our work provides a rare look at the impacts of habitat fragmentation on microarthropods and their interactions and demonstrates that the effects of habitat fragmentation can impact the constituent members of a multi-level trophic interaction without necessarily impacting the interaction itself.
Fragmentation effects on mite communities
Fragmentation effects influenced mites through edge creation, where mite abundance and richness were higher at patch edges regardless of patch connectivity or patch shape. This pattern is likely due to the more hospitable microclimate near edges in this landscape experiment. Our study system consists of open habitat patches surrounded by a woody matrix, resulting in forested edges that reduce sunlight, increase soil moisture and air humidity, and reduce temperatures, relative to the core part of the open habitats (Cadenasso et al. 1997; Chen et al. 1999; Ries et al. 2004; Tuff et al. 2016). Previous studies in our system have found that locations near edges have relatively lower temperatures, and therefore higher relative humidity (Johnson and Haddad 2011), which could reduce desiccation rates of the mites. Additionally, mite abundance has been shown to be positively correlated with humidity in landscape studies in agricultural settings (Çobanoğlu and Kumral 2016). Our work demonstrates that the diversity and abundance of small, wind-dispersed organisms, such as mites, which are commonly thought to be ubiquitous, are still affected by edges produced by fragmentation of habitat that is orders of magnitude larger than themselves.
Mite abundance and richness may have also been elevated near patch edges due to an influx of mites from the pine plantation matrix surrounding the landscape fragments in our experiment. The microclimate features of the matrix habitat are likely more suitable for mites in the same ways that locations near edges in fragments are (higher moisture, shade, lower temperatures). This observed abundance pattern may have been additionally exacerbated by mite populations being reduced in patches by the prescribed burns carried out the previous winter. Thus, edge effects may have resulted at least in part from recolonization of oaks in the spring and summer near edges to greater degrees than in patch centers, which would be reasonable if mites are weak dispersers. Data describing mite populations in the matrix and in patches over time would be needed to evaluate these influx and post-fire recolonization hypotheses.
This complication where the organisms that make up part of our studied interaction may be able to use the matrix habitat between our habitat patches may be a complicating factor in our findings. The habitat of the patches (longleaf pine savanna) differs from that of the matrix in many important ways. However, for the purpose of mites, a different habitat fragmentation framework may be more applicable, such as a framework that treats the broad landscape (both the patches and matrix) as part of a continuum, which has shown benefits in assessing species in some other systems (Fischer and Lindenmayer 2006; Brudvig et al. 2017). Our results are likely applicable to mites and fungi on leaves in longleaf pine savanna, and other forms of open habitat, but may change across different habitat conditions with greater canopy cover, such as those found in the matrix between our patches. Better understanding of similar systems may require additional and more explicit consideration of matrix habitats.
Compared to edge proximity, we found no evidence that patch shape or connectivity influenced mite abundance or richness. Arboreal mites in other systems disperse long distances (Lindo and Winchester 2009; Paynter et al. 2012). Species with dispersal capacities larger than the scale of habitat fragments may not respond strongly to connectivity or patch shape (Fletcher et al. 2018); and it is possible that such is the case for the mites in our experiment. If that is the case, then mites may more strongly respond to microclimatic features such as those associated with patch edges and respond less to large landscape structure. Indeed, previous work in this system with species that have strong dispersal capacities shows edges as having a greater effect than connectivity and patch shape (Sullivan et al. 2011).
Fragmentation effects and mite effects on leaf fungi
Leaf fungal abundance was impacted by edge proximity, with greater fungal abundance near edges. However, there were no impacts of connectivity nor patch shape on leaf fungal abundances. This result is supported by past work on abiotically-dispersed fungi in our system, which showed that connectivity and patch shape did not influence their abundance (Sullivan et al. 2011). Contrary to our expectation, blocking domatia, though effective in reducing mites, had no effect on leaf fungal abundance. This is similar to the findings of Monks et al. (2007), which similarly showed that blocking domatia did not affect foliar fungal density, albeit not in a fragmentation context. Though it is possible that mite abundances were generally too low to control fungal levels in our system, work on mite-fungal trophic interactions in other contexts has demonstrated that small shifts in mite abundance of a plant’s leaves can have large impacts on the fungal abundance on those leaves (Norton et al. 2000). Thus, it is possible that the mite densities that we observed could still be having a measurable effect on foliar fungal abundance. In spite of the lack of influence of mite abundance on fungus, both fungal abundance and mite abundance were higher at edges, likely due to favorable microclimate factors for both groups. Thus, fragmentation alters fungal abundance through the creation of edges due to bottom-up changes to environmental conditions, rather than top-down control by fungivorous mites. We also found no evidence that patch connectivity, patch shape, or edge proximity influenced mite-fungus interactions. As a result, although the effects of fragmentation affected the abundance of both mites and fungus, it did not alter trophic interactions between these groups.
Conclusion
As debate continues about the degree to which fragmentation and its effects matter for biodiversity (Fletcher et al. 2018; Fahrig et al. 2019), we provide experimental evidence that edges created by fragmentation alter the diversity and abundance of leaf-dwelling mites as well as the abundance of leaf fungi. These findings illustrate how cryptic aspects of biodiversity can be influenced by fragmentation, shedding light on novel fragmentation effects, and suggesting the need for new research to more broadly consider the consequences of fragmentation for understudied taxa.
Data availability
Our data is publicly archived at the Environmental Data Initiative (EDI). Mite genetic sequences are uploaded to GenBank.
References
Agrawal AA, Karban R (1997) Domatia mediate plant-arthropod mutualism. Nature 387:562–563. https://doi.org/10.1111/mms.12147
Aguilar R, Ashworth L, Galetto L, Aizen MA (2006) Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecol Lett 9:968–980. https://doi.org/10.1111/j.1461-0248.2006.00927.x
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Brooks ME, Kristensen K, van Benthem KJ et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400. https://doi.org/10.32614/rj-2017-066
Brouwer YM, Clifford HT (1990) An annotated list of domatia-bearing species. Notes from Jodrell Lab 12:1–33. https://doi.org/10.3/JQUERY-UI.JS
Brückmann SV, Krauss J, van Achterberg C, Steffan-Dewenter I (2011) The impact of habitat fragmentation on trophic interactions of the monophagous butterfly Polyommatus coridon. J Insect Conserv 15:707–714. https://doi.org/10.1007/s10841-010-9370-7
Brudvig LA, Leroux SJ, Albert CH et al (2017) Evaluating conceptual models of landscape change. Ecography (Cop) 40:74–84. https://doi.org/10.1111/ecog.02543
Cadenasso ML, Traynor MM, Pickett STA (1997) Functional location of forest edges: gradients of multiple physical factors. Can J For Res 27:774–782. https://doi.org/10.1139/x97-013
Chen J, Saunders SC, Crow TR et al (1999) Microclimate in forest ecosystem the effects and lanscape ecology. Bioscience 49:288–297
Çobanoğlu S, Kumral NA (2016) The biodiversity, density and population trend of mites (Acari) on Capsicum annuum L. in temperate and semi-arid zones of Turkey. Syst Appl Acarol 21:907–918. https://doi.org/10.11158/saa.21.7.5
Collinge SK (2000) Effects of grassland fragmentation on insect species loss, colonization, and movement patterns. Ecology 81:2211–2226. https://doi.org/10.1890/0012-9658
Damschen EI, Brudvig LA, Burt MA et al (2019) Ongoing accumulation of plant diversity through habitat connectivity in an 18-year experiment. Sci (80-) 365:1478–1480. https://doi.org/10.1126/science.aax8992
Didham RK, Kapos V, Ewers RM (2012) Rethinking the conceptual foundations of habitat fragmentation research. Oikos 121:161–170. https://doi.org/10.1111/j.1600-0706.2011.20273.x
Evans DM, Turley NE, Levey DJ, Tewksbury JJ (2012) Habitat patch shape, not corridors, determines herbivory and fruit production of an annual plant. Ecology 93:1016–1025. https://doi.org/10.1890/11-0642.1
Fahrig L, Arroyo-Rodríguez V, Bennett JR et al (2019) Is habitat fragmentation bad for biodiversity? Biol Conserv 230:179–186. https://doi.org/10.1016/j.biocon.2018.12.026
Fischer J, Lindenmayer DB (2006) Beyond fragmentation: the continuum model for fauna research and conservation in human-modified landscapes. Oikos 112:473–480. https://doi.org/10.1111/j.0030-1299.2006.14148.x
Fletcher RJ, Didham RK, Banks-Leite C et al (2018) Is habitat fragmentation good for biodiversity? Biol Conserv 226:9–15. https://doi.org/10.1016/j.biocon.2018.07.022
Ghazy NA, Suzuki T (2014) Desiccation tolerance in diapausing spider mites Tetranychus urticae and T. kanzawai (Acari: Tetranychidae). Exp Appl Acarol 63:49–55. https://doi.org/10.1007/s10493-013-9760-0
Gibbs JP, Stanton EJ (2001) Habitat fragmentation and arthropod community change: carrion beetles, phoretic mites, and flies. Ecol Appl 11:79–85. https://doi.org/10.1890/1051-0761(2001)011[0079:HFAACC]2.0.CO;2
Gilbert F, Gonzalez A, Evans-Freke I (1998) Corridors maintain species richness in the fragmented landscapes of a microecosystem. Proc R Soc B Biol Sci 265:577–582. https://doi.org/10.1098/rspb.1998.0333
Gonzalez A, Lawton JH, Gilbert FS et al (1998) Metapopulation dynamics, abundance, and distribution in a microecosystem. Science 281:2045–2047. https://doi.org/10.1126/science.281.5385.2045
Grostal P, O’Dowd DJ (1994) Plants, mites and mutualism: leaf domatia and the abundance and reproduction of mites on Viburnum tinus (Caprifoliaceae). Oecologia 97:308–315. https://doi.org/10.1007/BF00317319
Haddad NM, Bowne DR, Cunningham A et al (2003) Corridor use by diverse taxa. Ecology 84:609–615. https://doi.org/10.1890/0012-9658(2003)084[0609:CUBDT]2.0.CO;2
Haddad NM, Brudvig LA, Clobert J et al (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:1–9. https://doi.org/10.1126/sciadv.1500052
Harris JL (2000) Safe, low-distortion tape touch method for fungal slide mounts. J Clin Microbiol 38:4683–4684. https://doi.org/10.1128/jcm.38.12.4683-4684.2000
Huffaker CB (1958) Experimental Studies on Predation: Dispersion Factors and Predator-Prey Oscillations. Hilgardia 27:343–383
Johnson BL, Haddad NM (2011) Edge effects, not connectivity, determine the incidence and development of a foliar fungal plant disease. Ecology 92:1551–1558. https://doi.org/10.1890/10-1072.1
Jose S, Jokela EJ, Miller D (2006) The longleaf pine ecosystem: Ecology, silviculture, and restoration. Springer, New York City, New York
Kruess A, Tscharntke T (1994) Habitat fragmentation, species loss, and biological control. Sci (80-) 264:1581–1584. https://doi.org/10.1126/science.264.5165.1581
Laine AL, Hanski I (2006) Large-scale spatial dynamics of a specialist plant pathogen in a fragmented landscape. J Ecol 94:217–226. https://doi.org/10.1111/j.1365-2745.2005.01075.x
Lefcheck JS (2016) piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol Evol 7:573–579. https://doi.org/10.1111/2041-210X.12512
Levey DJ, Caughlin TT, Brudvig LA et al (2016) Disentangling fragmentation effects on herbivory in understory plants of longleaf pine savanna. Ecology 97:2248–2258. https://doi.org/10.1002/ecy.1466
Lindo Z, Winchester NN (2009) Spatial and environmental factors contributing to patterns in arboreal and terrestrial oribatid mite diversity across spatial scales. Oecologia 160:817–825. https://doi.org/10.1007/s00442-009-1348-3
Magrach A, Laurance WF, Larrinaga AR, Santamaria L (2014) Meta-analysis of the effects of forest fragmentation on interspecific interactions. Conserv Biol 28:1342–1348. https://doi.org/10.1111/cobi.12304
Martinson HM, Fagan WF (2014) Trophic disruption: a meta-analysis of how habitat fragmentation affects resource consumption in terrestrial arthropod systems. Ecol Lett 17:1178–1189. https://doi.org/10.1111/ele.12305
Monks A, O’Connell DM, Lee WG et al (2007) Benefits associated with the domatia mediated tritrophic mutualism in the shrub Coprosma lucida. Oikos 116:873–881. https://doi.org/10.1111/j.2007.0030-1299.15654.x
Mueller T, Lenz J, Caprano T et al (2014) Large frugivorous birds facilitate functional connectivity of fragmented landscapes. J Appl Ecol 51:684–692. https://doi.org/10.1111/1365-2664.12247
Norton AP, English-Loeb G, Belden E (2001) Host plant manipulation of natural enemies: Leaf domatia protect beneficial mites from insect predators. Oecologia 126:535–542. https://doi.org/10.1007/s004420000556
Norton AP, English-Loeb G, Gadoury D, Seem RC (2000) Mycophagous mites and foliar pathogens: Leaf domatia mediate tritrophic interactions in grapes. Ecology 81:490–499. https://doi.org/10.1890/0012-9658(2000)081[0490:MMAFPL]2.0.CO;2
O’Dowd DJ, Pemberton RW (1994) Leaf domatia in Korean plants: floristics, frequency, and biogeography. Vegetatio 114:137–148
O’Dowd DJ, Willson MF (1989) Leaf domatia and mites on Australasian plants: ecological and evolutionary implications. Biol J Linn Soc 37:191–236
O’Dowd DJ, Willson MF (1997) Leaf domatia and the distribution and abundance of foliar mites in broadleaf deciduous forest in Wisconsin. Am Midl Nat 137:337–348
Öckinger E, Schweiger O, Crist TO et al (2010) Life-history traits predict species responses to habitat area and isolation: a cross-continental synthesis. Ecol Lett 13:969–979. https://doi.org/10.1111/j.1461-0248.2010.01487.x
Onzo A, Sabelis MW, Hanna R (2010) Effects of ultraviolet radiation on predatory mites and the role of refuges in plant structures. Environ Entomol 39:695–701. https://doi.org/10.1603/EN09206
Paynter Q, Gourlay AH, Rolando CA, Watt MS (2012) Dispersal of the Scotch broom gall mite Aceria genistae: implications for biocontrol. New Zeal Plant Prot 65:81–84. https://doi.org/10.30843/nzpp.2012.65.5429
Pearse IS, LoPresti E, Schaeffer RN et al (2020) Generalising indirect defence and resistance of plants. Ecol Lett 23:1137–1152. https://doi.org/10.1111/ele.13512
Pemberton RW, Turner CE (1989) Occurrence of predatory and fungivorous mites in leaf domatia. Am J Bot 76:105–112
R Core Team (2021) R: a language and environment for statistical computing
Reed MB, Swanson M, Gaither S et al (2002) Changing Identity. Savannah River Site at 50. U.S. Government Publishing Office, Washington, DC
Ries L, Fletcher RJ, Battin J, Sisk TD (2004) Ecological responses to habitat edges: mechanisms, models, and variability explained. Annu Rev Ecol Evol Syst 35:491–522. https://doi.org/10.1146/annurev.ecolsys.35.112202.130148
Romero GQ, Benson WW (2005) Biotic interactions of mites, plants and leaf domatia. Curr Opin Plant Biol 8:436–440. https://doi.org/10.1016/j.pbi.2005.05.006
Sullivan LL, Johnson BL, Brudvig LA, Haddad NM (2011) Can dispersal mode predict corridor effects on plant parasites? Ecology 92:1559–1564
Thrall PH, Godfree R, Burdon JJ (2003) Influence of spatial structure on pathogen colonization and extinction: a test using an experimental metapopulation. Plant Pathol 52:350–361. https://doi.org/10.1046/j.1365-3059.2003.00863.x
Tuff KT, Tuff T, Davies KF (2016) A framework for integrating thermal biology into fragmentation research. Ecol Lett 19:361–374. https://doi.org/10.1111/ele.12579
Walter DE (1996) Living on leaves: Mites, tomenta, and leaf domatia. Annu Rev Entomol 41:101–114
Walter DE, O’Dowd DJ (1992) Leaves with domatia have more mites. Ecology 73:1514–1518. https://doi.org/10.2307/1940694
Walter DE, Proctor HC (2013) Mites: Ecology, Evolution, and Behavior, 2nd edn. Springer
Zabel J, Tscharntke T (1998) Does fragmentation of Urtica habitats affect phytophagous and predatory insects differentially? Oecologia 116:419–425. https://doi.org/10.1007/s004420050605
Acknowledgements
We thank the US Forest Service for the creation and maintenance of our experimental blocks. Particularly important have been John Blake, Ed Olson, Andy Horcher, Jim Segar, and the fire management crew. We would like to thank Traci Thomas and Melissa Burt for logistical support, Jameel Al-Haddad for supplies, Carlos Garcia-Robledo, Christina Baer, and Caroline Edwards for assistance developing the DNA barcoding procedure, and the Tar Elves for helping apply the mite-exclusion treatment. Thanks also to the Corridor Group and the Weber Lab for their thoughts in the formation of this project and feedback on drafts of this manuscript. Western science benefits and derives from a history of colonialism and racism, and working towards making reparations for these histories starts with acknowledging this past and present. The site where we worked (SRS) occupies the ancestral, traditional, and contemporary lands of the Westo, Edisto Natchez-Kusso, and Yamassee peoples. Additionally, in the formation of SRS in the 1950s, ~ 6000 people were forcibly displaced, the majority of whom were Black landowners, tenant farmers, and sharecroppers (Reed et al. 2002). Funding for this research was provided by the National Science Foundation (Awards: 1354085, 1819026, 1912729, 1913501, 1831164) and by the US Department of Energy to the US Department of Agriculture-Forest Service-Savannah River under Interagency Agreement DE-AI09-00SR22188.
Funding
Funding for this research was provided by the National Science Foundation (Awards: 1354085, 1819026, 1912729, 1913501, 1831164) and by the US Department of Energy to the US Department of Agriculture-Forest Service-Savannah River under Interagency Agreement DE-AI09-00SR22188.
Author information
Authors and Affiliations
Contributions
Ideas and design for this study were formulated by all authors. Data collection was performed by Carolyn Graham and Christopher Warneke. Analyses were performed by Christopher Warneke. Initial manuscript draft was written by Carolyn Graham and Christopher Warneke. All authors revised and edited the manuscript. Final manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Carolyn D. K. Graham and Christopher R. Warneke are co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Graham, C.D.K., Warneke, C.R., Weber, M. et al. The impact of habitat fragmentation on domatia-dwelling mites and a mite-plant-fungus tritrophic interaction. Landsc Ecol 37, 3029–3041 (2022). https://doi.org/10.1007/s10980-022-01529-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-022-01529-2