Abstract
Context
Forest management and disturbances cause habitat fragmentation for saproxylic species living on old-growth attributes. The degree of habitat spatiotemporal continuity required by these species is a key question for designing biodiversity-friendly forestry, and it strongly depends on species’ dispersal. The “stability–dispersal” model predicts that species using stable habitats should have lower dispersal abilities than species associated with ephemeral habitat and thus respond to habitat availability at smaller scales.
Objectives
We aimed at testing the stability–dispersal model by comparing the spatial scales at which saproxylic beetle guilds using substrates with contrasted stability (from stable to ephemeral: cavicolous, fungicolous, saproxylophagous and xylophagous guilds) are affected by landscape structure (i.e. habitat amount and aggregation).
Methods
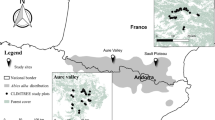
We sampled saproxylic beetles using a spatially nested design (plots within landscape windows). We quantified habitat availability (tree cavities, polypores and deadwood) in 1-ha plots, 26-ha buffers around plots and 506-ha windows, and analyzed their effect on the abundance and diversity of associated guilds.
Results
The habitat amount within plots and buffers positively affected the abundance of the cavicolous and the fungicolous guilds whereas saproxylophagous and xylophagous did not respond at these scales. The habitat aggregation within windows only positively affected the saproxylophagous species richness within plots and also on the similarity in species composition among plots.
Conclusions
Beetle guilds specialized on more stable habitat were affected by landscape structure at smaller spatial scales, which corroborated the stability–dispersal model. In managed forests, the spatial grain of conservation efforts should therefore be adapted to the target habitat lifetime.




Similar content being viewed by others
References
Barbosa P, Krischik V, Lance D (1989) Life-history traits of forest-inhabiting flightless Lepidoptera. Am Midl Nat 122(2):262–274
Barton Maintainer K (2018) Package “MuMIn”: multi-model inference. R-package version 1.40.4. https://cran.r-project.org/web/packages/MuMIn/
Baselga A, Orme D, Villeger S (2013) Package “betapart”: partitioning beta diversity into turnover and nestedness components. R-package version 1.2. https://cran.r-project.org/web/packages/betapart/
Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Scheipl F, Grothendieck G, Green P (2017) Package “lme4”: linear mixed-effects models using “Eigen” and S4. R-package version 1.1-15. https://cran.r-project.org/web/packages/lme4/
Bouget C, Brin A, Tellez D, Archaux F (2015) Intraspecific variations in dispersal ability of saproxylic beetles in fragmented forest patches. Oecologia 177(3):911–920.
Bouget C, Brustel H, Brin A, Noblecourt T (2008a) Sampling saproxylic beetles with window flight traps: methodological insights. Rev d’Ecologie (La Terre la Vie) 63:21–32
Bouget C, Brustel H, Zagatti P (2008b) The FRench Information system on Saproxylic BEetle Ecology (FRISBEE): an ecological and taxonomical database to help with the assessment of forest conservation status. Rev d’Ecologie (La Terre la Vie) suppl. 10: 3–36. hal-00454436f
Bouget C, Parmain G, Gilg O, Noblecourt T, Nusillard B, Paillet Y, Pernot C, Larrieu L, Gosselin F (2014) Does a set-aside conservation strategy help the restoration of old-growth forest attributes and recolonization by saproxylic beetles? Anim Conserv 17(4):342–353.
Boutin S, Hebert D (2002) Landscape ecology and forest management: developing an effective partnership. Ecol Appl 12(2):390–397
Brin A, Valladares L, Ladet S, Bouget C (2016) Effects of forest continuity on flying saproxylic beetle assemblages in small woodlots embedded in agricultural landscapes. Biodivers Conserv 25(3):587–602.
Cadotte MW (2006) Dispersal and species diversity: a meta-analysis. Am Nat 167(6):913–924.
D’Eon RGD, Glenn SM, Parfitt I, Fortin M (2002) Landscape connectivity as a function of scale and organism vagility in a real forested landscape. Conserv Ecol 6(2):10
Davies KF, Margules CR, Lawrence JF (2000) Which traits of species predict population declines in experimental forest fragments? Ecology 81(5):1450–1461
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev Camb Philos Soc 81:117–142.
Feldhaar H, Schauer B (2018) Dispersal of Saproxylic Insects. In: Ulyshen MD (ed) Saproxylic insects. Zoological monographs, vol 1. Springer, Cham, pp 515–546
Franc N, Götmark F, Økland B, Nordén B, Paltto H (2007) Factors and scales potentially important for saproxylic beetles in temperate mixed oak forest. Biol Conserv 135:86–98.
Franklin JF, Lindenmayer DB (2009) Importance of matrix habitats in maintaining biological diversity. Proc Natl Acad Sci 106(2):349–350.
Gibb H, Hjältén J, Ball JP, Pettersson RB, Landin J, Alvini O, Danell K (2006a) Wing loading and habitat selection in forest beetles: Are red-listed species poorer dispersers or more habitat-specific than common congenerics? Biol Conserv 132(2):250–260.
Gibb H, Hjältén J, Ball JP, Atlegrim O, Pettersson RB, Hilszczański J, Johansson T, Danell K (2006b) Effects of landscape composition and substrate availability on saproxylic beetles in boreal forests: a study using experimental logs for monitoring assemblages. Ecography 29(2):191–204.
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1(2):1–9.
Hanski I (1999) Habitat connectivity, habitat continuity, and metapopulations in dynamic landscapes. Oikos 87(2):209–219
Harrison RG (1980) Dispersal polymorphisms in insects. Annu Rev Ecol Syst 11(1):95–118.
Hedin J, Ranius T, Nilsson SG, Smith HG (2008) Restricted dispersal in a flying beetle assessed by telemetry. Biodivers Conserv 17(3):675–684.
Holland JD, Fahrig L, Cappuccino N (2005) Body size affects the spatial scale of habitat-beetle interactions. Oikos 110(1):101–108.
Jackson HB, Fahrig L (2012) What size is a biologically relevant landscape? Landsc Ecol 27(7):929–941.
Jackson HB, Fahrig L (2015) Are ecologists conducting research at the optimal scale? Glob Ecol Biogeogr 24(1):52–63.
Jacobsen RM, Sverdrup-Thygeson A, Birkemoe T (2015) Scale-specific responses of saproxylic beetles: combining dead wood surveys with data from satellite imagery. J Insect Conserv 19(6):1053–1062.
Jactel H, Gaillard J (1991) A preliminary study of the dispersal potential of Ips sexdentatus (Boern) (Col., Scolytidae) with an automatically recording flight mill. J Appl Entomol 112:138–145.
Janssen P, Fuhr M, Cateau E, Nusillard B, Bouget C (2017) Forest continuity acts congruently with stand maturity in structuring the functional composition of saproxylic beetles. Biol Conserv 205:1–10.
Jonsson M (2003) Colonisation ability of the threatened tenebrionid beetle Oplocephala haemorrhoidalis and its common relative Bolitophagus reticulatus. Ecol Entomol 28(2):159–167.
Jonsson M, Johannesen J, Seitz A (2003) Comparative genetic structure of the threatened tenebrionid beetle Oplocephala haemorrhoidalis and its common relative Bolitophagus reticulatus. J Insect Conserv 7(2):111–124.
Komonen A (2008) Colonization experiment of fungivorous beetles (Ciidae) in a lake-island system. Entomol Tidskr 129:141–145
Komonen A, Müller J (2018) Dispersal ecology of deadwood organisms and connectivity conservation. Conserv Biol 32(3):535–545.
Kouki J, Löfman S, Martikainen P, Rouvinen S, Uotila A (2001) Forest fragmentation in Fennoscandia: linking habitat requirements of wood-associated threatened species to landscape and habitat changes. Scand J For Res suppl. 3:27–37.
Larrieu L, Cabanettes A, Delarue A (2012) Impact of silviculture on dead wood and on the distribution and frequency of tree microhabitats in montane beech-fir forests of the Pyrenees. Eur J For Res 131(3):773–786.
Larrieu L, Cabanettes A, Brin A, Bouget C, Deconchat M (2014a) Tree microhabitats at the stand scale in montane beech–fir forests: practical information for taxa conservation in forestry. Eur J For Res 133(2):355–367.
Larrieu L, Cabanettes A, Gonin P, Lachat T, Paillet Y, Winter S, Bouget C, Deconchat M (2014b) Deadwood and tree microhabitat dynamics in unharvested temperate mountain mixed forests: a life-cycle approach to biodiversity monitoring. For Ecol Manage 334:163–173.
Larrieu L, Cabanettes A, Gouix N, Burnel L, Bouget C, Deconchat M (2017) Development over time of the tree-related microhabitat profile: the case of lowland beech–oak coppice-with-standards set-aside stands in France. Eur J For Res 136(1):37–49.
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7(7):601–613.
Lenoir J, Hattab T, Pierre G (2017) Climatic microrefugia under anthropogenic climate change: implications for species redistribution. Ecography 40(2):253–266.
Levin SA (1992) The problem of pattern and scale in ecology. Ecology 73(6):1943–1967
Lombardi F, Lasserre B, Tognetti R, Marchetti M (2008) Deadwood in relation to stand management and forest type in Central Apennines (Molise, Italy). Ecosystems 11(6):882–894.
Mandelbrot B (1983) Fractals and the geometry of nature, vol 173. WH Freeman, New-York
McMullin RT, Wiersma YF (2019) Out with OLD growth, in with ecological continNEWity: new perspectives on forest conservation. Front Ecol Environ 17(3):176–181.
Mestre L, Jansson N, Ranius T (2018) Saproxylic biodiversity and decomposition rate decrease with small-scale isolation of tree hollows. Biol Conserv 227:226–232.
Miguet P, Jackson HB, Jackson ND, Martin AE, Fahrig L (2016) What determines the spatial extent of landscape effects on species? Landsc Ecol 31(6):1177–1194.
Müller J, Goßner MM (2010) Three-dimensional partitioning of diversity informs state-wide strategies for the conservation of saproxylic beetles. Biol Conserv 143(3):625–633.
Nordén B, Appelqvist T (2001) Conceptual problems of ecological continuity and its bioindicators. Biodivers Conserv 10(5):779–791.
Paillet Y, Pernot C, Boulanger V, Debaive N, Fuhr M, Gilg O, Gosselin F (2015) Quantifying the recovery of old-growth attributes in forest reserves: a first reference for France. For Ecol Manage 346:51–64.
Ranius T (2006) Measuring the dispersal of saproxylic insects: a key characteristic for their conservation. Popul Ecol 48(3):177–188.
Ranius T, Kindvall O (2006) Extinction risk of wood-living model species in forest landscapes as related to forest history and conservation strategy. Landsc Ecol 21(5):687–698.
Ranius T, Niklasson M, Berg N (2009) Development of tree hollows in pedunculate oak (Quercus robur). For Ecol Manage 257(1):303–310.
Ranius T, Johansson V, Fahrig L (2011a) Predicting spatial occurrence of beetles and pseudoscorpions in hollow oaks in southeastern Sweden. Biodivers Conserv 20(9):2027–2040.
Ranius T, Martikainen P, Kouki J (2011b) Colonisation of ephemeral forest habitats by specialised species: beetles and bugs associated with recently dead aspen wood. Biodivers Conserv 20(13):2903–2915.
Rubene D, Schroeder M, Ranius T (2015) Diversity patterns of wild bees and wasps in managed boreal forests: effects of spatial structure, local habitat and surrounding landscape. Biol Conserv 184:201–208.
Saint-Germain M, Drapeau P (2011) Response of saprophagous wood-boring beetles (Coleoptera: Cerambycidae) to severe habitat loss due to logging in an aspen-dominated boreal landscape. Landsc Ecol 26(4):573–586.
Saura S, Pascual-Hortal L (2007) A new habitat availability index to integrate connectivity in landscape conservation planning: comparison with existing indices and application to a case study. Landsc Urban Plan 83(2):91–103.
Seibold S, Bässler C, Brandl R, Fahrig L, Förster B, Heurich M, Hothorn T, Scheipl F, Thorn S, Müller J (2017) An experimental test of the habitat-amount hypothesis for saproxylic beetles in a forested region. Ecology 98(6):1613–1622.
Southwood TRE (1977) Habitat, the templet for ecological strategies? J Anim Ecol 46(2):336–365
Stokland JN, Siitonen J, Jonsson BG (2012) Biodiversity in dead wood. Cambridge University Press, Cambridge
Thuiller W, Pollock LJ, Gueguen M, Münkemüller T (2015) From species distributions to meta-communities. Ecol Lett 18(12):1321–1328.
Travis JMJ, Dytham C (1999) Habitat persistence, habitat availability and the evolution of dispersal. Proc R Soc B Biol Sci 266(1420):723–728.
Villard MA, Metzger JP (2014) Beyond the fragmentation debate: a conceptual model to predict when habitat configuration really matters. J Appl Ecol 51(2):309–318.
Wiens JA (1989) Spatial scaling in ecology. Funct Ecol 3(4):385–397
With KA (1997) The application of neutral models in conservation biology. Conserv Biol 11(5):1069–1080
Acknowledgements
We are grateful to Carl Moliard and Benoit Nusillard for their considerable field assistance. Our grateful thanks are also extended to Thierry Noblecourt, Fabien Soldati, Thomas Barnouin and Guilhem Parmain, as well as Oliver Rose (Ciidae), Yves Gomy (Histeridae) and Benoit Nusillard (Latriidae, Curculionidae), for their valuable contribution to insect identification. We are also grateful to the agents from the National Forest Office involved in this project, particularly Vincent Boulanger, Michel Leblanc and Marguerite Delaval for their assistance in the project logistics and the provision of forest management data, as well as Nicolas Caillé and Nicolas Hilt for insect trap collection. We thank Laurent Larrieu for his helpful advice in field sampling of tree-related microhabitats, Vicki Moore for checking the English language and three anonymous reviewers for their constructive comments on previous versions of the manuscript. This work was funded by the National Research Institute of Science and Technology for Environment and Agriculture (IRSTEA) and the National Forest Office (ONF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Percel, G., Laroche, F. & Bouget, C. The scale of saproxylic beetles response to landscape structure depends on their habitat stability. Landscape Ecol 34, 1905–1918 (2019). https://doi.org/10.1007/s10980-019-00857-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-019-00857-0