Abstract
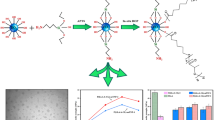
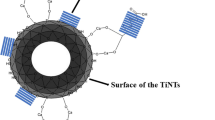
This work presents the surface functionalization of titanium dioxide nanoparticles with 1H-1-carboxylate of isopropyl-imidazole and 3-aminopropyltrimethoxysilane. The surface and thermal properties of titanium dioxide are characterized by nuclear magnetic resonance, Fourier-transform infrared spectroscopy, zeta potential, X-ray diffraction, and differential scanning calorimetry. The functionalization mentioned above improves the dispersibility of titanium dioxide in polymeric matrices such as polylactic acid. Polylactic acid compounds are extruded with these nanoparticles; their crystallization capacity is studied by non-isothermal crystallization. The results obtained indicate the successful binding of organic structures to titanium dioxide. Likewise, the dispersion improves when the nanoparticle is silanized, reducing agglomeration when the isopropyl-imidazole 1H-1-carboxylate is bound to the organosilicon coating. DSC measurements show that isopropyl imidazole 1H-1-carboxylate adhered to the organosilicon coating exhibits excellent thermal stability up to 300 °C. Finally, the photo-degradation of the composites is studied by Fourier-transform infrared spectroscopy and atomic force microscopy, showing that the use of isopropyl-imidazole 1H-1-carboxylate inhibits the degradation of the polylactic acid composite compared to the pure polymer.















Similar content being viewed by others
References
Diebold U. The surface science of titanium dioxide. Surf Sci Rep. 2003;48:53–229.
Shiraishi F, Ueno M, Chand R, Shibata Y, Luitel HN. Effect of silanization of titanium dioxide on photocatalytic decomposition of 2,4-dinitropheonol under irradiation with artificial UV light and sunlight. J Chem Technol Biotechnol. 2014;89:81–7.
Solís-Gómez A, Neira-Velázquez MG, Morales J, Sánchez-Castillo MA, Pérez E. Improving stability of TiO2 particles in water by RF-plasma polymerization of poly(acrylic acid) on the particle surface. Colloids Surf A Physicochem Eng Aspects. 2014;451:66–74.
González-Rodríguez V, Lizeth Zapata-Tello D, Vallejo-Montesinos J, Zárraga Núñez R, Gonzalez-Calderon JA, Pérez E. Improving titanium dioxide dispersion in water through surface functionalization by a dicarboxylic acid. J Dispers Sci Technol. 2018;40:1–7.
Bonhôte P, Gogniat E, Grätzel M, Ashrit PV. Novel electrochromic devices based on complementary nanocrystalline TiO2 and WO3 thin films. Thin Solid Films. 1999;350:269–75.
Altan M, Yildirim H. Mechanical and morphological properties of polypropylene and high density polyethylene matrix composites reinforced with surface modified nano sized TiO2 particles. Int J Mater Metall Eng. 2010;4:231–7.
Gonzalez-Calderon JA, Vallejo-Montesinos J, Almendarez-Camarillo A, Montiel R, Pérez E. Non-isothermal crystallization analysis of isotactic polypropylene filled with titanium dioxide particles modified by a dicarboxylic acid. Thermochim Acta [Internet]. 2016;631:8–17. https://doi.org/10.1016/j.tca.2016.03.007.
Yang C, Zhu B, Wang J, Qin Y. Structural changes and nano-TiO2 migration of poly(lactic acid)-based food packaging film contacting with ethanol as food simulant. Int J Biol Macromol. 2019;139:85–93.
Ramesan MT, Sampreeth T. In situ synthesis of polyaniline/Sm-doped TiO2 nanocomposites: evaluation of structural, morphological, conductivity studies and gas sensing applications. J Mater Sci Mater Electron. 2018;29:4301–11.
Suhailath K, Ramesan MT. Investigations on the structural, mechanical, thermal, and electrical properties of Ce-doped TiO2 /poly(n-butyl methacrylate) nanocomposites. J Therm Anal Calorim. 2019;135:2159–69.
Suhailath K, Ramesan MT. Effect of nano-ce-doped TiO2 on AC conductivity and DC conductivity modeling studies of poly (n-butyl methacrylate). J Electron Mater. 2018;47:6484–93.
Kaseem M, Hamad K, Rehman ZU. Review of recent advances in polylactic acid/TiO2 composites. Materials. 2019;12:3659.
Tu-morn M, Pairoh N, Sutapun W, Trongsatitkul T. Effects of titanium dioxide nanoparticle on enhancing degradation of polylactic acid/low density polyethylene blend films. Mater Today Proc. 2019;17:2048–61.
Henderson MA. The interaction of water with solid surfaces: fundamental aspects revisited. Surf Sci Rep. 2002;46:1–308.
Clayton J. Pigment/dispersant interactions in water-based coatings. Surf Coat Int. 1997;80:414–20.
Vallejo-Montesinos J, Muñoz UM, Gonzalez-Calderon JA. Mechanical properties, crystallization and degradation of polypropylene due to nucleating agents, fillers and additives. Polypropyl Propert Uses Benefits. 2016;4:83–140.
Todesco RV, Ergenc N, Stabilizers L, Stabilization L. Addit Plast Appl. 2002;56:225–38.
Balabanovich AI, Klimovtsova IA, Prokopovich VP, Prokopchuk NR. Thermal stability and thermal decomposition study of hindered amine light stabilizers. Thermochim Acta. 2007;459:1–8.
Maringer L, Roiser L, Wallner G, Nitsche D, Buchberger W. The role of quinoid derivatives in the UV-initiated synergistic interaction mechanism of HALS and phenolic antioxidants. Polym Degrad Stab. 2016;131:91–7.
Zhang W, Liu D, Hua C, Zhao J, Chen B, Gou X. Synthesis and properties of multifunctional nitroxyl radical hindered-amine light stabilizers. Heterocycl Commun. 2015;21:77–81.
Reingruber E, Buchberger W. Analysis of polyolefin stabilizers and their degradation products. J Sep Sci. 2010;33:3463–75.
Step EN, Turro NJ, Gande ME, Klemchuk PP. Mechanism of polymer stabilization by hindered-amine light stabilizers (HALS) Model investigations of the interaction of peroxy radicals with HALS amines and amino ethers. Macromolecules. 1994;27:2529–39.
Gijsman P. New synergists for hindered amine light stabilizers. Polymer (Guildf). 2002;43:1573–9.
Chinelatto MA, Agnelli JAM, Canevarolo SV. Synthesis and photostabilizing performance of a polymeric HALS based on 1,2,2,6,6-pentamethylpiperidine and vinyl acetate. Polímeros. 2015;25:575–80.
Zhao J, Milanova M, Warmoeskerken MMCG, Dutschk V. Surface modification of TiO2 nanoparticles with silane coupling agents. Colloids Surf A Physicochem Eng Aspects. 2012;413:273–9. https://doi.org/10.1016/j.colsurfa.2011.11.033.
Ahangaran F, Navarchian AH. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: a review. Adv Colloid Interface Sci. 2020;286:102298.
Fuad MYA, Ismail Z, Ishak ZAM, Omar AKM. Application of rice husk ash as fillers in polypropylene: effect of titanate, zirconate and silane coupling agents. Eur Polymer J. 1995;31:885–93.
Liu Y, Guo L, Wang W, Sun Y, Wang H. Modifying wood veneer with silane coupling agent for decorating wood fiber/high-density polyethylene composite. Construct Build Mater. 2019;224:691–9.
Zheng W, Tang C, Xie J, Gui Y. Micro-scale effects of nano-SiO2 modification with silane coupling agents on the cellulose/nano-SiO2 interface. Nanotechnology. 2019;30:445701.
Ismail H, Mega L, Abdul Khalil HPS. Effect of a silane coupling agent on the properties of white rice husk ash-polypropylene/natural rubber composites. Polym Int. 2001;50:606–11.
Zheng J, Han D, Ye X, Wu X, Wu Y, Wang Y, et al. Chemical and physical interaction between silane coupling agent with long arms and silica and its effect on silica/natural rubber composites. Polymer (Guildf). 2018;135:200–10.
You S-L, Kelly JW. Highly efficient enantiospecific synthesis of imidazoline-containing amino acids using bis(triphenyl)oxodiphosphonium trifluoromethanesulfonate. Organ Lett Am Chem Soc. 2004;6:1681–3.
Wu Y-Q, Limburg DC, Wilkinson DE, Hamilton GS. Formation of nitrogen-containing heterocycles using di(imidazole-1-yl)methanimine. J Heterocycl Chem. 2009;40:191–3.
Yavari I, Nematpour M, Ghanbari E. A tandem synthesis of 5-sulfonylimino-2-imidazolones from sulfonoketenimides and dialkyl azodicarboxylates. Mol Divers. 2014;18:721–5.
Alfonso M, Tárraga A, Molina P. Pyrrole, imidazole, and triazole derivatives as ion-pair recognition receptors. Tetrahedron Lett. 2016;57:3053–9.
Biron E, Chatterjee J, Kessler H. Solid-phase synthesis of 1,3-azole-based peptides and peptidomimetics. Organ Lett Am Chem Soc. 2006;8:2417–20.
Li M, Zhang W, Wang W, He Q, Yin M, Qin X, et al. Imidazole improves cognition and balances Alzheimer’s-like intracellular calcium homeostasis in transgenic Drosophila model. Neurourol Urodyn. 2017;37:1250–7.
Maru MS and MKS. Synthesis, Characterization and antimicrobial evaluation of transition metal complexes of monodentate 2-(substituted phenyl)-1h-benzo [d] imidazoles. 2015;42:216–27
Borhade AV, Tope DR, Gite SG. Synthesis, characterization and catalytic application of silica supported tin oxide nanoparticles for synthesis of 2,4,5-tri and 1,2,4,5-tetrasubstituted imidazoles under solvent-free conditions. Arab J Chem. 2017;10:S559–67.
Zhang S, Ye J, Sun Y, Kang J, Liu J, Wang Y, et al. Electrospun fibrous mat based on silver (I) metal-organic frameworks-polylactic acid for bacterial killing and antibiotic-free wound dressing. Chem Eng J. 2020;390:124523.
Li C, Ma C, Li J. Highly efficient flame retardant poly(lactic acid) using imidazole phosphate poly(ionic liquid). Polym Adv Technol. 2020;31:1765–75.
Liu S, Qin S, He M, Zhou D, Qin Q, Wang H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos Part B Eng. 2020;199:108238.
Zhong M, Zhang X, Yang DH, Zhao B, Xie Z, Zhou Z, et al. Zeolitic imidazole framework derived composites of nitrogen-doped porous carbon and reduced graphene oxide as high-efficiency cathode catalysts for Li-O2 batteries. Inorgan Chem Front. 2017;4:1533–8.
Lunt J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym Degrad Stab. 1998;3910:145–52.
Díaz-Pedraza A, Piñeros Castro Y, Ro T. Bi-layer materials based on thermoplastic corn starch, polylactic acid and modifiedpolypropylene. Revista Mexicana de Ingeniería Química. 2013;12:505–11.
Sun Z, Zhang L, Liang D, Xiao W, Lin J. Mechanical and thermal properties of PLA biocomposites reinforced by coir fibers. Int J Polym Sci. 2017;2017:1–9.
Elsawy MA, Kim KH, Park JW, Deep A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew Sustain Energy Rev. 2017;79:1346–52.
Lonkar SP, Kushwaha OS, Leuteritz A, Heinrich G, Singh RP. Self photostabilizing UV-durable MWCNT/polymer nanocomposites. RSC Adv. 2012;2:12255.
Papadopoulos L, Terzopoulou Z, Zamboulis A, Klonos PA, Bikiaris DN, Kyritsis A, et al. Effects of Ag, ZnO and TiO2 nanoparticles at low contents on the crystallization, semi-crystalline morphology, interfacial phenomena and segmental dynamics of PLA. Mater Today Commun. 2021;27:102192.
Terzopoulou Z, Klonos PA, Kyritsis A, Tziolas A, Avgeropoulos A, Papageorgiou GZ, et al. Interfacial interactions, crystallization and molecular mobility in nanocomposites of poly (lactic acid) filled with new hybrid inclusions based on graphene oxide and silica nanoparticles. Polymer (Guildf). 2019;166:1–12.
Wang XJ, Huang Z, Wei MY, Lu T, Nong DD, Zhao JX, et al. Catalytic effect of nanosized ZnO and TiO2 on thermal degradation of poly(lactic acid) and isoconversional kinetic analysis. Thermochim Acta. 2019;672:14–24.
Li W, Zhang C, Chi H, Li L, Lan T, Han P, et al. Development of antimicrobial packaging film made from poly(lactic acid) incorporating titanium dioxide and silver nanoparticles. Molecules. 2017;22:1170.
Raquez JM, Habibi Y, Murariu M, Dubois P. Polylactide (PLA)-based nanocomposites. Prog Polym Sci. 2013;38:1504–42.
Amza CG, Zapciu A, Baciu F, Vasile MI, Popescu D. Aging of 3D printed polymers under sterilizing UV-C radiation. Polymers. 2021;13:1–16.
Kuo CFJ, Lan WL, Chen CY, Tsai HC. Property modification and process parameter optimization design of polylactic acid composite materials. Part I: polylactic acid toughening and photo-degradation modification and optimized parameter design. Textile Res J. 2015;85:13–25.
Can U, Kaynak C. Performance of polylactide against UV irradiation : synergism of an organic UV absorber with micron; 2020.
Gonzalez-Calderon JA, Vallejo-Montesinos J, Martínez-Martínez HN, Cerecero-Enríquez R, López-Zamora L. Effect of chemical modification of titanium dioxide particles via silanization under properties of chitosan/potato-starch films. Revista Mexicana de Ingeniería Química. 2019;18:913–27.
Avrami M. Kinetics of phase change II. Transformation-time relations for random distribution of nuclei. J Chem Phys. 1940;8:212–24.
Avrami M. Granulation, phase change, and microstructure kinetics of phase change III. J Chem Phys. 1941;9:177–84.
Avrami M. Kinetics of phase change. I: general theory. J Chem Phys. 1939;7:1103–12.
Jeziorny A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by d.s.c. Polymer (Guildf). 1978;19:1142–4.
Liu T, Mo Z, Zhang H. Nonisothermal crystallization behavior of a novel poly(aryl ether ketone): PEDEKmK. J Appl Polym Sci. 1998;67:815–21.
Wang J, Dou Q. Non-isothermal crystallization kinetics and morphology of isotactic polypropylene (iPP) nucleated with rosin-based nucleating agents. J Macromol Sci Part B Phys. 2007;46:987–1001.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci Part C Polym Sympos. 2007;6:183–95. https://doi.org/10.1002/polc.5070060121.
Ek S, Iiskola EI, Niinisto L. Atomic layer deposition of amino-functionalized silica surfaces using N-(2-aminoethyl)-3-aminopropyltrimethoxysilane as a silylating agent. J Phys Chem B. 2004;108:9650–5.
López-Zamora L, Martínez-Martínez HN, González-Calderón JA. Improvement of the colloidal stability of titanium dioxide particles in water through silicon based coupling agent. Mater Chem Phys. 2018;217:285–90.
Ijadpanah-saravi H, Safari M, Khodadadi-darban A. Synthesis of titanium dioxide nanoparticles for photocatalytic degradation of cyanide in wastewater. Anal Lett. 2014;47:1772–82.
Van Cong D, Trang NTT, Giang NV, Lam TD, Hoang T. Effect of TiO2-crystal forms on the photo-degradation of EVA/PLA blend under accelerated weather testing. J Electron Mater. 2016;45:2536–46.
Nim B, Sreearunothai P, Opaprakasit P, Petchsuk A. Preparation and properties of electrospun fibers of titanium dioxide-loaded polylactide/polyvinylpyrrolidone blends. Appl Sci Eng Prog. 2019;12:52–8.
Rokbani H, Ajji A. Rheological properties of poly(lactic acid) solutions added with metal oxide nanoparticles for electrospinning. J Polym Environ. 2018;26:2555–65.
Zhang Q, Lei H, Cai H, Han X, Lin X, Qian M, et al. Improvement on the properties of microcrystalline cellulose/polylactic acid composites by using activated biochar. J Clean Prod. 2020;252:119898.
Luo Y, Cao Y, Guo G. Effects of TiO2 nanoparticles on the photo-degradation of poly(lactic acid). J Appl Polym Sci. 2018;135:1–8.
Therias S, Larché JF, Bussière PO, Gardette JL, Murariu M, Dubois P. Photochemical behavior of polylactide/ZnO nanocomposite films. Biomacromol. 2012;13:3283–91.
Copinet A, Bertrand C, Govindin S, Coma V, Couturier Y. Effects of ultraviolet light (315 nm), temperature and relative humidity on the degradation of polylactic acid plastic films. Chemosphere. 2004;55:763–73.
Marra A, Cimmino S, Silvestre C. Effect of TiO2 and ZnO on PLA degradation in various media. Adv Mater Sci. 2017;2:1–8.
Acknowledgements
The authors want to thank CONACyT for funding the project CF2019 265239, which made this work possible. J.A. Gonzalez-Calderon thank to CONACYT by the support of the Catedras CONACYT program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
González Calderón, J.A., Austria Gutiérrez, A., Sanchez, G. et al. Improvement in the physicochemical properties of titanium dioxide by surface modification with 1H-1-carboxylate of isopropyl-imidazole and 3-aminopropyltrimethoxysilane: case study—as a filler in polylactic acid composites. J Therm Anal Calorim 147, 12365–12382 (2022). https://doi.org/10.1007/s10973-022-11476-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11476-4