Abstract
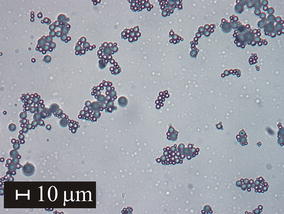
The ytterbium aluminum garnet composition YbAG (62.5 mol.% Al2O3, 37.5 mol.% Yb2O3) was prepared in the form of glass microspheres by flame synthesis. Precursor powder for flame synthesis with high homogeneity was prepared by modified sol–gel Pechini method. XRD pattern of prepared glass microspheres indicated predominantly amorphous nature of the sample. Detailed study of morphology of the microspheres by scanning electron microscopy revealed the presence of a small fraction of partially or fully crystallized microspheres. The high-temperature X-ray powder diffraction analysis (HT XRD) was carried out in the temperature interval 750–1450 °C: The temperature dependence of phase composition was determined. Crystallization of Yb3Al5O12—ytterbium aluminum garnet phase—was observed in the temperature range 900–1200 °C. The DSC analysis with heating rates 2, 4, 6, 8, 10 °C min−1 in temperature interval 25–1200 °C was performed in N2 atmosphere to study thermal behavior and crystallization kinetics of prepared glass microspheres. The two exothermic effects at 918 and 939 °C were observed, which were attributed to Yb3Al5O12 crystallization. The crystallization kinetics of prepared sample was examined with the use of JMAK model, and the kinetic triplet—frequency factor A = (1.8 ± 2.2) 10+28 min−1 (for the first peak), A = (1.2 ± 1.6) 10+55 min−1 (for the second peak), apparent activation energy Eapp = (6.4 ± 0.1) 10+02 kJ mol−1 (for the first peak), Eapp = (1.3 ± 0.1) 10+03 kJ mol−1 (for the second peak) and the Avrami coefficient m = 3 (for the first peak) and m = 2 (for the second peak)—was determined using RSS, R2adj , AIC and WAIC criteria.






Similar content being viewed by others
References
Nørby P, Jensen KMO, Lock N, Christensen M, Iversen BB. Continuous flow supercritical water synthesis and temperature-dependent defect structure analysis of YAG and YbAG nanoparticles. Cryst Growth Des. 2016;16:2646–52.
Xu X, Zhao Z, Song P, Jiang B, Zhou G, Xu J, et al. Optical spectroscopy of Yb3Al5O12 single crystal. Spectrochim Acta Part A Mol Biomol Spectrosc. 2005;62:645–8.
Li C, Xu J, Liu W, Lin H, Liu Y, Wang D, et al. Synthesis and characterization of Er:Yb3Al5O12 nanopowder. Russ J Phys Chem A. 2015;89:2263–6.
Marquardt H, Speziale S, Jahn S, Ganschow S, Schilling FR. Single-crystal elastic properties of (Y,Yb)3Al5O12. J Appl Phys. 2009;106:093519.
Prnová A, Plško A, Valúchová J, Švančárek P, Klement R, Michálková M, et al. Crystallization kinetics of yttrium aluminate glasses. J Therm Anal Calorim. 2018;133:227–36.
Clarke DR, Levi CG. Materials design for the next generation thermal barrier coatings. Annu Rev Mater Res. 2003;33:383–417.
Zhou Y, Xiang H, Feng Z. Theoretical investigation on mechanical and thermal properties of a promising thermal barrier material: Yb3Al5O12. J Mater Sci Technol. 2014;30:631–8.
Moncorgé R, Camy P, Doualan JL, Braud A, Margerie J, Ramirez LP, et al. Pure and Yb3+ doped fluorites (Ca, Sr, Ba)F2: a renewal for the future high intensity laser chains. J Lumin. 2013;133:276–81.
Chénais S, Druon F, Forget S, Balembois F, Georges P. On thermal effects in solid-state lasers: the case of ytterbium-doped materials. Prog Quantum Electron. 2006;30:89–153.
Prnová A, Bodišová K, Klement R, Migát M, Veteška P, Škrátek M, et al. Preparation and characterization of Yb2O3–Al2O3 glasses by the Pechini sol-gel method combined with flame synthesis. Ceram Int. 2014;40:6179–84.
Wilding MC. Aluminates. In: Ceramic and glass materials. Boston: Springer; 2008. p. 49–70.
Dubnikova N, Garskaite E, Beganskiene A, Kareiva A. Sol–gel synthesis and characterization of sub-microsized lanthanide Ho, Tm, Yb, Lu aluminium garnets. Opt Mater Amst. 2011;33:1179–84.
Wu Y, Li J, Pan Y, Liu Q, Guo J. Synthesis of nano-sized Yb3Al5O12 powders by the urea co-precipitation method. Ceram Int. 2009;35:25–7.
Tratsiak Y, Bokshits Y, Korjik M, Tamulaitis G, Trusova E, Vaitkevičius A. Garnet-based complex substituted glass ceramic materials. Radiat Meas. 2019;122:97–100.
Tratsiak Y, Trusova E, Bokshits Y, Korjik M, Vaitkevičius A, Tamulaitis G. Garnet-type crystallites, their isomorphism and luminescence properties in glass ceramics. Cryst Eng Commun. 2019;21:687–93.
Rosenflanz A, Frey M, Endres B, Anderson T, Richards E, Schardt C. Bulk glasses and ultrahard nanoceramics based on alumina and rare-earth oxides. Nature. 2004;430:761–4.
Reis ST, Kim CW, Brow RK, Ray CS. Nucleation and crystallization as induced by bending stress in lithium silicate glass fibers. J Non-Cryst Solids. 2004;348:1.
Reben M, Kosmal M, Ziabka M, Pichniarczyk P, Grelowska I. The influence of TiO2 and ZrO2 on microstructure and crystallization behaviour of CRT glass. J Non-Cryst Solids. 2015;425:118.
Ray CS, Day DE. Identifying internal and surface crystallisation by differential thermal analysis for the glass-to-crystal transformation. Thermochim Acta. 1996;280(281):163–74.
McMillan PF, Wilding MC. Direct density determination of low-and high density glassy polymorphs following a liquid–liquid phase transition in Y2O3–Al2O3 supercooled liquids. J Non-Cryst Solids. 2008;354:1015–25.
Reis ST, Pascual MJ, Brow RK, Ray CS, Zhang T. Crystallisation and processing of SOFC sealing glasses. J Non-Cryst Solids. 2010;356:3009–12.
Li W, Mitchell BS. Nucleation and crystallisation in calcium aluminate glasses. J Non-Cryst Solids. 1999;255:199–207.
Zahedi M, Roohpour N, Ray AK. Kinetic study of crystallisation of sol-gel derived calcia–alumina binary compounds. J Alloys Compd. 2014;582:277–82.
Dohnalová Ž, Šulcová P, Gorodylova N. Study of ceramic pigments based on cobalt doped Y2O3–Al2O3 system. J Therm Anal Calorim. 2014;116:647–54.
Černá A, Chromčíková M, Macháček J, Hruška B, Liška M. Viscosity and configuration entropy of glasses for CHROMPIC vitrification. J Therm Anal Calorim. 2018;133:365–70.
McGilvery CM, Gent SD, Payzant EA, MacKenzie M, Craven AJ, McComb DV. Investigation of crystallization processes from hafnium silicate powders prepared from an oxychloride sol–gel. J Am Ceram Soc. 2012;95:3985–91.
Serantoni M, Costa AL, Lanelli Ch, Esposito L. Crystallization behaviour of Yb-doped and undoped YAG nanoceramics synthesized by microwave-assisted urea precipitation. Ceram Int. 2014;40:11837–44.
Prnová A, Klement R, Bodišová K, Valúchová J, Galusek D, Bruneel E, et al. Thermal behaviour of yttrium aluminate glasses studied by DSC, high-temperature X-ray diffraction, SEM and SEM–EDS. J Therm Anal Calorim. 2017;128:1407–15.
Prnová A, Plško A, Klement R, Valúchová J, Haladejová K, Švančárek P, et al. Crystallization kinetics of binary La2O3–Al2O3 glass. J Non-Cryst Solids. 2018;501:55–61.
Prnová A, Plško A, Valúchová J, Haladejová K, Klement R, Galusek D. Crystallization kinetics of glass microspheres with yttrium aluminium garnet (YAG) composition. J Therm Anal Calorim. 2018;131:1115–23.
Pechini MP. Method of preparing lead and alkaline-earth titanates and niobates and coating method using the same to form a capacitor. U.S. Patent No. 3,330,697, 1967.
Šesták J, Šimon P, editors. Thermal analysis of micro, nano- and non-crystalline materials: transformation, crystallization, kinetics and thermodynamics. London: Springer; 2013. p. 225–46.
Kolmogorov AE. On the statistic theory of metal crystallization. Izv Akad Nauk SSSR Ser Mat. 1937;1:355–9 (in Russian).
Johnson WA, Mehl RF. Reaction kinetics in processes of nucleation and growth. Trans Am Inst Min Metall Pet Eng. 1939;135:416–58.
Tanaka H. Thermal analysis and kinetics of solid state reactions. Thermochim Acta. 1995;267:29–44.
Šesták J, Šatava V, Wendlandt WW. The study of heterogeneous processes by thermal analysis. Thermochim Acta. 1973;7:333–556.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetic Committee recommendation for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Akaike H. Information theory and an extension of maximum likelihood principle. In: Petrov BN, Csáki F, editors. 2nd international symposium on information theory. Budapest: Akadémia Kiadó; 1973. p. 267–81.
Akaike H. A new look at the statistical model identification. IEEE Trans Automat Control. 1974;19:716–23.
Kim HJ, Cavanaugh JE. Model selection criteria based on Kullback information measures for nonlinear regression. J Stat Plan Infer. 2005;134:332–49.
Johnson JB, Omland KS. Model selection in ecology and evolution. Trends Ecol Evol. 2004;19:101–8.
Roduit B, Hartmann M, Folly P, Sarbach A, Baltensperger R. Prediction of thermal stability of materials by modified kinetic and model selection approaches based on limited amount of experimental points. Thermochim Acta. 2014;579:31–9.
Fabrichnaya O, Seifert HJ. Thermodynamic assessment of the ZrO2–Yb2O3–Al2O3 system. CALPHAD Comput Coupl Phase Diagr Termochem. 2010;34:206–11.
Acknowledgements
This paper is a part of dissemination activities of project FunGlass. This project has received funding from the European Union’s Horizon 2020, research and innovation programme under Grant Agreement No. 739566. The financial support of this work by the projects APVV 0014-15, APVV-17-0049, VEGA 1/0527/18, VEGA 2/0088/16, VEGA 1/0064/18 and VEGA 2/0026/17 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prnová, A., Plško, A., Valúchová, J. et al. Crystallization kinetics of binary Yb2O3–Al2O3 glass. J Therm Anal Calorim 142, 2141–2148 (2020). https://doi.org/10.1007/s10973-020-10049-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10049-7