Abstract
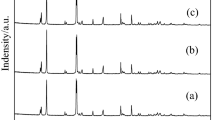
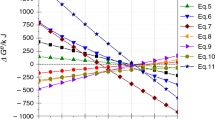
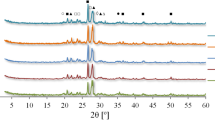
Jarosite method is most widely employed to remove iron through the zinc hydrometallurgical process, and ammonium jarosite sediment is produced. The low-temperature thermal decomposition of the ammonium jarosite sediment is desirable to recover natural resources and protect the environment. Herein, we aimed to study the influence of troilite addition on thermal decomposition of hydrothermally synthesized ammonium jarosite. The ammonium jarosite gets decomposed at 300 °C and results in a hematite crystal phase. The hematite, pyrite and magnetite phases have been observed at 400 °C. Furthermore, the hematite and magnetite are present as major phases and pyrrhotite phase has been detected as the minor phase at 500 °C. In addition, the amount of magnetite and hematite exhibited linear and inverse relationships with heat treatment temperature, respectively, in the temperature range of 500–800 °C. Also, the sulfur has been completely decomposed during the jarosite process after roasting at 500 °C and the estimated activation energy value (Ea) of 503.4 kJ mol−1 has been obtained from \(\ln (v/T_{\text{p}}^{2} )\) versus 1/Tp plots. Troilite can improve sulfur elimination from ammonium jarosite at low temperature.









Similar content being viewed by others
References
Senapati PK, Mishra BK. Rheological characterization of concentrated jarosite waste suspensions using Couette & tube rheometry techniques. Powder Technol. 2014;263:58–65.
Salinas E, Roca A, Cruells M, et al. Characterization and alkaline decomposition–cyanidation kinetics of industrial ammonium jarosite in NaOH media. Hydrometallurgy. 2001;60:237–46.
Kerolli-Mustafa M, Bacic I, Urkovic LC. Investigation of jarosite process tailing waste by means of raman and infrared spectroscopy. Materialwiss Werkstofftech. 2013;44:768–73.
Katsioti M, Tsakiridis PE, Agatzini-Leonardou TS, et al. Examination of the jarosite–alunite precipitate addition in the raw meal for the production of Portland and sulfoaluminate-based cement clinkers. Int J Miner Process. 2005;76:217–24.
Ju S, Zhang Y, Zhang Y, et al. Clean hydrometallurgical route to recover zinc, silver, lead, copper, cadmium and iron from hazardous jarosite residues produced during zinc hydrometallurgy. J Hazard Mater. 2011;192:554–8.
Han H, Sun W, Hu Y, et al. Anglesite and silver recovery from jarosite residues through roastingand sulfidization-flotation in zinc hydrometallurgy. J Hazard Mater. 2014;278:49–54.
Ristic M, Music S, Orehovec Z, et al. Thermal decomposition of synthetic ammonium jarosite. J Mol Struct. 2005;744–747:295–300.
Wen X, Liang Y, Bai P, et al. First-principles calculations of the structural, elastic and thermodynamic properties of mackinawite (FeS) and pyrite (FeS2). Phys B. 2017;525:119–26.
Bolin TB. S-XANES analysis of thermal iron sulfide transformations in a suite of argonne premium coals: a study of particle size effects during pyrolysis. Int J Coal Geol. 2014;131:200–13.
Malenga EN, Mulaba-Bafubiandi AF, Nheta W. Alkaline leaching of nickel bearing ammonium jarosite precipitate using KOH, NaOH and NH4OH in the presence of EDTA and Na2S. Hydrometallurgy. 2015;155:69–78.
Spratt H, Rintoul L, Avdeev M, Wayde M. The thermal decomposition of hydronium jarosite and ammoniojarosite. J Therm Anal Calorim. 2014;115:101–9.
Frost RL, Wain DL, Wills R-A, et al. A thermogravimetric study of the alunites of sodium, potassium and ammonium. Thermochim Acta. 2006;443:56–61.
Taylor P, Rummery TE, Owen DG. Reactions of iron monosulfide solids with aqueous hydrogen sulfide up to 160 °C. J Inorg Nucl Chem. 1979;41:1683–7.
Huang F, Zhang L, Yi B, et al. Effect of H2O on pyrite transformation behavior during oxy-fuel combustion. Fuel Process Technol. 2015;131:458–65.
Tang C, Wang H, Dong S, et al. Study of SO2 effect on selective catalytic reduction of NOx with NH3 over Fe/CNTs: the change of reaction route. Catal Today. 2018;307:2–11.
Ma X, Tan H, Liu J, et al. Preparation of ammonium jarosite and estimated activation energy of thermal decomposition in reducing atmosphere. J Therm Anal Calorim. 2019;135:2565–72.
Maraden A, Stojan P, Matyas R, et al. Impact of initial grain temperature on the activation energy and the burning rate of cast double-base propellant. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7927-y.
Suekkhayad A, Noisong P, Danvirutai C. Synthesis, thermodynamic and kinetic studies of the formation of LiMnPO4 from a new Mn(H2PO2)2·H2O precursor. J Therm Anal Calorim. 2017;129:123–34.
Hussain AI, Palani A, Aitani AM, et al. Catalytic cracking of vacuum gasoil over -SVR, ITH, and MFI zeolites as FCC catalyst additives. Fuel Process Technol. 2017;161:23–32.
Ogunbadejo B, Aitani A, Čejka J, et al. The effect of alkylation route on ethyltoluene production over different structural types of zeolites. Chem Eng J. 2016;306:1071–80.
Acknowledgements
This work was supported by the Research Fund of the Sichuan Science and Technology Program of China (2017GZ0401), Xinjiang Science and Technology Program of China (2017E0207) and Natural Science Foundation of Southwest University of Science and Technology (18zx7101).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, X., Tan, H., Dong, F. et al. Influence of troilite on the decomposition of ammonium jarosite and estimated activation energy. J Therm Anal Calorim 139, 933–939 (2020). https://doi.org/10.1007/s10973-019-08480-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08480-6