Abstract
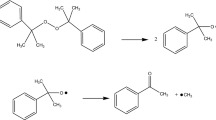
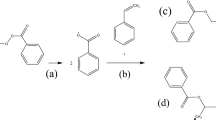
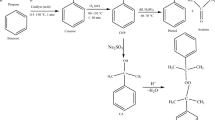
In this paper, we describe thermokinetic properties and decomposition characteristics of benzoyl peroxide, dicumyl peroxide, and lauroyl peroxide, which are widely used in the polymerization process as energy boosters. In the past, many accidents occurred that involved overpressure and runaway excursion of the process and thermal explosion. One reason for accidents is because of the peroxy group (–O–O–) of organic peroxides (OPs) due to its thermal instability and high sensitivity when exposing to heat. Apparent activation energy and pre-exponential factor were obtained during decomposition via non-isothermal well-recognized kinetic equation, fitting curve tests, and approximate solution to design safer reaction conditions when OPs are used as fuel. Moreover, the storage conditions were investigated for the simulation of thermal explosion in a 24-kg cubic box package and a 400-kg barrel reactor for commercial application. Experimental results established the novel features of solid explosion hazard of OPs.













Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor (s−1)
- CT:
-
Control temperature (°C)
- C p :
-
Specific heat capacity (J g−1 °C−1)
- E a :
-
Apparent activation energy (kJ mol−1)
- ET:
-
Emergency temperature (°C)
- i :
-
Component number
- k 0 :
-
Pre-exponential factor
- k 1, k 2 :
-
Rate constants of a reaction or stage
- NC:
-
Number of components
- n :
-
Unit outer normal on the boundary
- n 1, n 2 :
-
Reaction orders of a specific stage
- q :
-
Heat flow rate (W)
- R :
-
Gas constant (J mol−1 K−1)
- r :
-
Reaction rate constant (mol l−1 s−1)
- SADT:
-
Self-accelerating decomposition temperature (°C)
- T :
-
Temperature of sample (K)
- TCL:
-
Time to conversion limit (day)
- T cr :
-
Critical temperature (°C)
- T 0 :
-
Exothermic onset temperature (°C)
- T f :
-
Final temperature (K)
- T p :
-
Peak temperature (°C)
- t :
-
Time (min)
- TMRiso :
-
Time to maximum rate under isothermal conditions (day)
- W :
-
Heat power (J s−1)
- z:
-
Autocatalytic constant
- ∆H d :
-
Heat of decomposition (J g−1)
- α :
-
Degree of conversion of a component
- β :
-
Scanning rate (°C min−1)
- ρ :
-
Density (kg m−3)
References
Dou Z, Jiang JC, Wang ZR, Zhao SP, Yang HQ, Mao GB. Kinetic analysis for spontaneous combustion of sulfurized rust in oil tanks. J Loss Prev Process Ind. 2014;32:387–92.
Dou Z, Jiang JC, Zhao SP, Zhang WX, Ni L, Zhang MG, Wang ZR. Analysis on oxidation process of sulfurized rust in oil tank. J Therm Anal Calorim. 2017;128:125–34.
Liu SH, Shu CM, Hou HY. Applications of thermal hazard analyses on process safety assessments. J Loss Prev Process Ind. 2015;33:59–69.
Duh YS. Chemical kinetics on thermal decompositions of cumene hydroperoxide in cumene studied by calorimetry: an overview. Thermochim Acta. 2016;637:102–9.
Liu SH, Hou HY, Chen JW, Weng SY, Lin YC, Shu CM. Effects of thermal runaway hazard for three organic peroxides conducted by acids and alkalines with DSC, VSP2, and TAM III. Thermochim Acta. 2013;566:226–32.
Lv JY, Chen WH, Chen LP, Tian YT, Yan JJ. Thermal risk evaluation on decomposition processes for four organic peroxides. Thermochim Acta. 2014;589:11–8.
Lizarraga E, Zabaleta C, Palop JA. Thermal behavior of quinoxaline 1,4-di-N-oxide derivatives. J Therm Anal Calorim. 2017;127:1655–61.
Chen WC, Shu CM. Prediction of thermal hazard for TBPTMH mixed with BPO through DSC and isoconversional kinetics analysis. J Therm Anal Calorim. 2016;126:1937–45.
Yamamoto YK, Miyake A. Influence of a mixed solvent containing ionic liquids on the thermal hazard of the cellulose dissolution process. J Therm Anal Calorim. 2017;127:743–8.
Zhang GY, Jin SH, Li LJ, Li YK, Wang DQ, Li W, Zhang T, Shu QH. Thermal hazard assessment of 4,10-dinitro-2,6,8,12-tetraoxa-4,10-diazaisowutrzitane (TEX) by accelerating rate calorimeter (ARC). J Therm Anal Calorim. 2016;126:467–71.
Tseng JM, Lin CP, Hung ST, Hsu J. Kinetic and safety parameters analysis for 1,1,-di-(tert-butylperoxy)-3,3,5-trimethylcyclohexane in isothermal and non-isothermal conditions. J Hazard Mater. 2011;192:1427–36.
Ho TC, Duh YS, Chen JR. Case studies of incidents in runaway reactions and emergency relief. Process Saf Prog. 1998;17:259–62.
Li XR, Koseki H, Iwata Y, Mok YS. Decomposition of methyl ethyl ketone peroxide and mixture with sulfuric acid. J Loss Prev Process Ind. 2004;17:23–8.
Liu SH, Hou HY, Shu CM. Thermal hazard evaluation of autocatalytic reaction for benzoyl peroxide by DSC and TAM III. Thermochim Acta. 2015;605:68–76.
Luo KM, Chang JG, Lin SH, Chang CT, Yeh TF, Hu KH, Kao CS. The criterion of critical runaway and stable temperatures in cumene hydroperoxide reaction. J Loss Prev Process Ind. 2001;14:229–39.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc. 1965;38:1881–6.
Flynn JH, Wall LA. J Res Natl Bur Stand Phys Chem. 1966;70:487–92.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci. 1966;4:323–8.
Lin CP, Tseng JM. Green technology for improving process manufacturing design and storage management of organic peroxide. Chem Eng J. 2012;180:284–92.
Chen JR, Cheng SY, Yuan MH, Kossoy AA, Shu CM. Hierarchical kinetic simulation for autocatalytic decomposition of cumene hydroperoxide at low temperatures. J Therm Anal Calorim. 2009;96:751–8.
You ML, Liu MY, Wu SH, Chi JH, Shu CM. Thermal explosion and runaway reaction simulation of lauroyl peroxide by DSC tests. J Therm Anal Calorim. 2009;96:777–82.
Lin CP, Tseng JM, Chang YM, Liu SH, Shu CM. Modeling liquid thermal explosion reactor containing tert-butyl peroxybenzoate. J Therm Anal Calorim. 2010;102:587–95.
Lin CP, Chang CP, Chou YC, Chu YC, Shu CM. Modeling solid thermal explosion containment on reactor HNIW and HMX. J Hazard Mater. 2010;176:549–58.
Kossoy AA, Shienman IY. Comparative analysis of the method for SADT determination. J Hazard Mater. 2007;143:626–38.
Hou HY, Shu CM, Duh YS. Exothermic decomposition of cumene hydroperoxide at low temperature conditions. AIChE J. 2001;47:1893–6.
STARe Software with Solaris Operating System. Operating instructions. Stockholm: Mettler Toledo; 2017.
You ML, Tseng JM, Liu MY, Shu CM. Runaway reaction of lauroyl peroxide with nitric acid by DSC. J Therm Anal Calorim. 2010;102:535–9.
Liu SH, Lin CP, Shu CM. Thermokinetic parameters and thermal hazard evaluation for three organic peroxides by DSC and TAM III. J Therm Anal Calorim. 2011;106:165–72.
Thermal safety software (TSS). St Petersburg Russia: ChemInform Saint-Petersburg (CISP) Ltd. 2016. http://www.cisp.spb.ru.
Kossoy AA, Benin AI, Akhmetshin YG. An advanced approach to reactivity rating. J Hazard Mater. 2005;118:9–17.
Kossoy AA, Akhmetshin YG. Identification of kinetic models for the assessment of reaction hazards. Process Saf Prog. 2007;26:209–20.
Kossoy AA, Hofelich T. Methodology and software for reactivity rating. Process Saf Prog. 2003;22:235–40.
Lu KT, Chen TC, Hu KH. Investigation of the decomposition reaction and dust explosion characteristics of crystalline benzoyl peroxides. J Hazard Mater. 2009;161:246–56.
Zaman F, Beezer AE, Mitchell JC, Clarkson Q, Elliot J, Davis AF, Willson RJ. The stability of benzoyl peroxide by isothermal microcalorimetry. Int J Pharm. 2001;277:133–7.
Lu KT, Chen TC, Hu KH. Investigation of the decomposition reaction and dust explosion characteristics of crystalline dicumyl peroxide. Process Saf Environ Prot. 2010;88:356–65.
Wu SH, Shyu ML, I YP, Chi JH, Shu CM. Evaluation of runaway reaction for dicumyl peroxide in a batch reactor by DSC and VSP2. J Loss Prev Process Ind. 2009;22:721–7.
Duh YS, Wu XH, Kao CS. Hazard ratings for organic peroxides. Process Saf Prog. 2008;27:89–99.
Frank-Kamenetskii DA. Diffusion and heat exchange in chemical kinetics. Princeton: Princeton University; 1955.
Frank-Kamenetskii DA. Diffusion and heat exchange in chemical kinetics. 2nd ed. New York, USA: Plenum Press; 1969.
Material safety data sheet (2007) Akzo Nobel Polymer Chemicals Bv. P.O. Box 247, 3800 AE Amersfoort, Netherlands
Recommendations on the transport of dangerous goods, manual of tests and criteria, 4th rev edn. New York, USA (2003)
Acknowledgements
The authors are indebted to the donors of the Anhui University of Science and Technology in China under the contract number QN201613 for financial support and appreciate Dr. Frank Oreovicz for editing the manuscript and providing useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, YF., Liu, SH., Shu, CM. et al. Energy estimation and modeling solid thermal explosion containment on reactor for three organic peroxides by calorimetric technique. J Therm Anal Calorim 130, 1201–1211 (2017). https://doi.org/10.1007/s10973-017-6717-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6717-2