Abstract
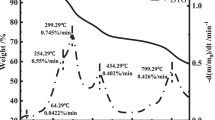
The paper focuses on the oxidation process of sulfurized rust in crude oil tank. Firstly, one sort of rust was put into the sulfurization and oxidation experimental apparatus. The chemical compositions and phase of sulfurized rust were analyzed by energy-dispersive X-ray spectrometer–scanning electron microscope technique. The result shows that the main contents are S, Fe and O and give a short length of side and diamond appearance, and a large pore size in structure. The oxidation of sulfurized rust at ambient temperature was investigated, which transferred from electrochemical reactions to chemical reactions. The result of thermal decomposition experiment indicates that the product of electrochemical reactions is ferrous sulfate. Hereafter, the thermo-gravimetric/differential scanning calorimetric (TG/DSC) technique was used to evaluate the self-heating hazards of pre-oxygenized sulfurized rust. The given TG/DSC curves at different heating rates are similar. Every curve consisted of three weightlessness stages and two weight gain stages. The corresponding apparent activation energy values, most probable kinetic model functions and pre-exponential factor values were calculated by the Flynn–Wall–Ozawa method, the Achar–Brindley–Sharp–Wendworth method and the Kissinger method. The final results described the complexity of oxidation process of pre-oxygenized sulfurized rust.









Similar content being viewed by others
References
Zheng S, Chen L, Chen C. Failure analysis of an A333Gr6 pipeline after exposure to a hydrogen sulfide environment. Eng Fail Anal. 2013;35:516–23.
Walker R. Instability of iron sulfides on recently excavated artifacts. Stud Conserv. 2001;46(2):141–52.
Walker R, Steele AD, Morgan TDB. The formation of pyrophoric iron sulphide from rust. Surf Coat Technol. 1987;31(2):183–97.
Li P, Wang S, Zhang Z. Study on the effect of water on the formation and pyrophoricity of ferrous sulfide. Pet Sci Technol. 2011;29(18):1922–31.
Li P, Li JD, Zhao SL. Prevention of spontaneous combustion of sulfur-containing oil tanks. Pet Sci Technol. 2006;24(9):1009–17.
Zhao S, Wang C, Li P, Ding D, Wan X. The influence of sulfurization of rust in oil tanks. Energy Sour Part A. 2007;29(12):1111–9.
Walker R, Steele AD, Morgan D. Deactivation of pyrophoric iron sulfides. Ind Eng Chem Res. 1997;36(9):3662–7.
Walker R, Steele AD, Morgan D. Pyrophoric nature of iron sulfides. Ind Eng Chem Res. 1996;35(5):1747–52.
Walker R, Steele AD, Morgan TDB. Pyrophoric oxidation of iron sulphide. Surf Coat Technol. 1988;34(2):163–75.
Hughes RI, Morgan TDB, Wilson RW. Is pyrophoric iron sulphide a possible source of ignition? Nature. 1974;248(5450):670.
Zhao SP, Jiang JC, Zheng J. Thermal analysis on kinetics of thermal decomposition of FeS. J Chongqing Univ. 2011;34(1):140–4.
Li P, Li JD, Zhao SL. Research on the danger of fires in oil tanks with sulfur. Fire Saf J. 2005;40(4):331–8.
Dou Z, Jiang JC, Wang Z. Kinetic analysis for spontaneous combustion of sulfurized rust in oil tanks. J Loss Prev Process Ind. 2014;32:387–92.
Hu RZ, Gao SL, Zhao FQ, Shi QZ, Zhang TL, Zhang JJ. Kinetics of thermal analysis. 2nd ed. Beijing: Science Publishing House; 2008 (in Chinese).
Ozawa T. Thermal analysis-review and prospect. Thermochim Acta. 2000;355(1):35–42.
Wagner M. Application handbook thermal analysis: thermal analysis in practice. Schwerzenbach: Mettler Toled; 2009.
Wierzejewska-Hnat M, Schriver A, Schriver-Mazzuoli L. FT infrared study of sulfur dioxide dimer. I. Nitrogen matrix. Chem Phys. 1994;183:117–26.
Kolta GA, Aska MH. Thermal decomposition of some metal sulphates. Thermochim Acta. 1975;11:65–72.
Li ZJ. Investigation on the mechanism of spontaneous combustion of sulphide ores and the key technologies for preventing fire. Changsha: Central South University; 2007.
Zhu FL, Feng QQ, Xu YF, Liu RT, Li KJ. Kinetics of pyrolysis of ramie fabric wastes from thermogravimetric data. J Therm Anal Calorim. 2015;119(1):651–7.
Zhang CF, Liu XD, Cheng J, Zhang JY. Study on curing kinetics of diglycidyl 1,2-cyclohexane dicarboxylate epoxy/episulfide resin system with hexahydro-4-methylphthalic anhydride as a curing agent. J Therm Anal Calorim. 2015;120(3):1893–903.
Criado JM, Gonzalez F, Morales J. Application of programmed temperature decomposition to the study of solid decomposition reactions taking place through the prout and tompkins mechanism. Thermochim Acta. 1975;12:337–42.
Yan L, He B, Hao T. Thermogravimetric study on the pressurized hydropyrolysis kinetics of a lignite coal. Int J Hydrog Energy. 2014;39(15):7826–33.
Xu Y, Zhang YF, Zhang GJ, Guo YF, Zhang J, Li GQ. Pyrolysis characteristics and kinetics of two Chinese low-rank coals. J Therm Anal Calorim. 2015;122(2):975–84.
Farjas J, Roura P. Exact analytical solution for the Kissinger equation: determination of the peak temperature and general properties of thermally activated transformations. Thermochim Acta. 2014;598:51–8.
Bai Y, Yang P, Zhang S, Li YQ, Gu Y. Curing kinetics of phenolphthalein-aniline-based benzoxazine investigated by non-isothermal differential scanning calorimetry. J Therm Anal Calorim. 2015;120(3):1755–64.
Shi N, Dou Q. Non-isothermal cold crystallization kinetics of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/treated calcium carbonate composites. J Therm Anal Calorim. 2015;119(1):635–42.
Tripathi M, Kumar D, Rajagopal C, Roy PK. Curing kinetics of self-healing epoxy thermosets. J Therm Anal Calorim. 2015;119(1):547–55.
Yi J, Zhao F, Wang B. Thermal behaviors, nonisothermal decomposition reaction kinetics, thermal safety and burning rates of BTATz-CMDB propellant. J Hazard Mater. 2010;181(1–3):432–9.
Acknowledgements
The authors are grateful for the support given by key project of National Natural Science Foundation of China Under Grant No. 21436006, National Natural Science Foundation of China Under Grant No. 51176070, PHC-CaiYuanpei (“Havu-Risk: Chemical industrial plants and domino effect: hazards, vulnerability, risks and sustainability” 32114TE, 2014-2016), the Priority Academic Program Development of Jiangsu Higher Education Institutions of China and the Graduate Education Innovation Project of Jiangsu Province Under Grant No. CXLX13_440.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dou, Z., Jiang, J.C., Zhao, S.P. et al. Analysis on oxidation process of sulfurized rust in oil tank. J Therm Anal Calorim 128, 125–134 (2017). https://doi.org/10.1007/s10973-016-5884-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5884-x