Abstract
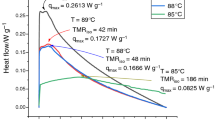
Organic peroxide (OP) has been applied in the industry for at least 40 years. Driven by significant investments in today’s high-profile energy, petrochemical, and polymer industries, the market and applications of OPs are expanding rapidly. However, the self-reactive nature has led to continuous research work on this topic. Nevertheless, tremendous progress has been made in hazard analysis, which has improved existing protocols or led to the discovery of new safety methods. OPs still cause related chemical hazards, and the limitations related to process hazards remain to be resolved. There is a lack of comprehensive systematic analysis of the process hazards of a wide variety of OPs. Different OPs, namely BPO, LPO, and the emerging OP, HTP-65W, were selected for investigation with several calorimetry techniques based on thermokinetic and heat transfer models. Determination methods have a crucial role in an operator for obtaining a systematic understanding of hazard properties under different process conditions, which is related to avoiding the occurrence of process disasters. For example, LPO has a shorter TMRad and TCL (< 1 min) than BPO and HTP-65W, indicating that LPO can be classified as an obvious hazardous material. Moreover, SADT < 25 °C can be used for evaluating LPO’s cooling system efficiency.









Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor of the Arrhenius equation (s−1)
- A (α):
-
Pre-exponential factor at conversion (s−1)
- A′(α):
-
Amended pre-exponential factor by a product of \(A (\alpha )\) and \(f (\alpha )\) (s−1)
- C p :
-
Specific heat capacity (J g−1 K−1)
- CT :
-
Control temperature (°C)
- E a :
-
Apparent activation energy (kJ mol−1)
- E(α):
-
Apparent activation energy factor at conversion (kJ mol−1)
- ET :
-
Emergency temperature (°C)
- f(α):
-
Kinetics function (dimensionless)
- i :
-
Component number (dimensionless)
- n :
-
Reaction order (dimensionless)
- n 1, n 2 :
-
Reaction orders of a specific stage (dimensionless)
- Q∞ i:
-
Reaction calorific effect (W)
- q :
-
Heat flow rate (W)
- R :
-
Gas constant (J mol−1 K−1)
- r :
-
Reaction rate constant (mol L−1 s−1)
- SADT :
-
Self-accelerating decomposition temperature (°C)
- t :
-
Time (min)
- T :
-
Temperature of sample (K)
- TCL :
-
Time to conversion limit (day, hr or min)
- T 0 :
-
Apparent exothermic onset temperature (°C)
- TMR :
-
Time to maximum rate (day)
- TMR ad :
-
Time to maximum rate under adiabatic conditions (day)
- TMR iso :
-
Time to maximum rate under isothermal conditions (day)
- U :
-
Heat transfer coefficient (W m−2 K−1)
- W :
-
Heat generation (J s−1)
- x :
-
Unit outer normal on the boundary (dimensionless)
- z :
-
Autocatalytic constant (dimensionless)
- ∆H d :
-
Heat of decomposition (J g−1)
- α :
-
Degree conversion of a component (dimensionless)
- β :
-
Heating rate (°C min−1)
- λ :
-
Heat conductivity (W m−1 K−1)
- ρ :
-
Density (kg m−3)
References
Murray R, Snowberg DR, Berry DS, Beach R, Rooney, SA, Swan D, Manufacturing a 9-meter thermoplastic composite wind turbine blade. National Renewable Energy Lab. (NREL), Golden, CO (United States). 2017.
Oliveira MCC, Diniz Cardoso ASA, Viana MM, Lins VDFC. The causes and effects of degradation of encapsulant ethylene vinyl acetate copolymer (EVA) in crystalline silicon photovoltaic modules: a review. Renew Sustain Energy Rev. 2018;81:2299–317.
Hejna A, Klein M, Saeb MR, Formela K. Towards understanding the role of peroxide initiators on compatibilization efficiency of thermoplastic elastomers highly filled with reclaimed GTR. Polym Test. 2019;73:143–51.
Przybysz M, Marć M, Klein M, Saeb MR, Formela K. Structural, mechanical and thermal behavior assessments of PCL/PHB blends reactively compatibilized with organic peroxides. Polym Test. 2018;67:513–21.
AkzoNobel. Nouryon completes further peroxides expansions; reports growth in first annual results. Addit Polym. 2019;2019:6–7.
AkzoNobel. Nouryon to double capacity for solvent-based organic peroxides in Mexico. Addit Polym. 2019;2019:9.
Chervin S, Bodman GT. Method for estimating decomposition characteristics of energetic chemicals. Process Saf Prog. 2003;22:241–3.
Levin M, Gonzales N, Zimmerman L, Yang J. Kinetics of acid-catalyzed cleavage of cumene hydroperoxide. J Hazard Mater. 2006;130:88–106.
Talouba IB, Balland L, Mouhab N, Abdelghani-Idrissi M. Kinetic parameter estimation for decomposition of organic peroxides by means of DSC measurements. J Loss Prev Process Ind. 2011;24:391–6.
Wang SY, Kossoy AA, Yao YD, Chen LP, Chen WH. Kinetics-based simulation approach to evaluate thermal hazards of benzaldehyde oxime by DSC tests. Thermochim Acta. 2017;655:319–25.
Wilberforce J. The use of the accelerating rate calorimeter to determine the SADT of organic peroxides. Milton Keynes: Columbia Scientific Industries; 1981.
Dannley R. Organic peroxides: their formation and reactions (Hawkins, E. G. E.). J Chem Educ. 1963;40:A62.
Wang Q, Rogers WJ, Mannan MS. Thermal risk assessment and rankings for reaction hazards in process safety. J Therm Anal Calorim. 2009;98:225–33.
SpecialChem, Organic peroxides market to reach $1069.1 M by 2022, Focus Surfactants, 2017;4.
Dizay H, Lau D, Nottage WM. Benzoyl peroxide and clindamycin topical skin preparation decreases Propionibacterium acnes deep colonization in shoulder arthroscopy. J Shoulder Elbow Surg. 2017;26:162.
Sparavigna A, Tenconi B, De Ponti I, La Penna L. An innovative approach to the topical treatment of acne. Clin Cosmet Investig Dermatol. 2015;8:179–85.
AkzoNobel. AkzoNobel strengthens global organic peroxides business with Mexican expansion. Addit Polym. 2017;2017:9.
AkzoNobel. AkzoNobel expands Mexican organic peroxide capacity, plans further increase in China. Addit Polym. 2017;2017:5–6.
United Nations. Recommendations on the transport of dangerous goods: model regulations (Twentieth Revised Edition). New York: United Nations Publications; 2018.
Di SI, Marotta R, Andreozzi R, Caprio V. Kinetic and chemical characterization of thermal decomposition of dicumylperoxide in cumene. J Hazard Mater. 2011;187:157–63.
Hou HY, Duh YS, Lee WL, Shu CM. Hazard evaluation for redox system of cumene hydroperoxide mixed with inorganic alkaline solutions. J Therm Anal Calorim. 2009;95:541–5.
Steensma M, Schuurman P, Malow M, Krause U, Wehrstedt KD. Evaluation of the validity of the UN SADT H.4 test for solid organic peroxides and self-reactive substances. J Hazard Mater. 2005;117:89–102.
Wang YW, Duh YS, Shu CM. Evaluationof adiabatic runaway reaction and vent sizing for emergency relief from DSC. J Therm Anal Calorim. 2006;85:225–34.
Lin CP, Tseng JM, Chang YM, Liu SH, Cheng YC, Shu CM. Modeling liquid thermal explosion reactor containing tert-butyl peroxybenzoate. J Therm Anal Calorim. 2010;102:587–95.
Zhu Y, Chen Y, Zhang L, Li W, Huang B, Wu J. Numerical investigation and dimensional analysis of reaction runaway evaluation for thermal polymerization. Chem Eng Res Des. 2015;104:32–41.
Huang YH. I YP, Chen NC, Wu SH, Horng JJ, Wu YT, Wen IJ, Thermal runaway reaction evaluation of benzoyl peroxide using calorimetric approaches. J Therm Anal Calorim. 2013;113:595–8.
Opfermann J, Hädrich W. Prediction of the thermal response of hazardous materials during storage using an improved technique. Thermochim Acta. 1995;263:29–50.
Wu KW, Hou HY, Shu CM. Thermalphenomena studies for dicumyl peroxide at various concentrations by DSC. J Therm Anal Calorim. 2006;83:41–4.
Zhang Y, Ni L, Jiang J, Jiang J, Zhang W, Jiang J, Zhang M. Thermal hazard analyses for the synthesis of benzoyl peroxide. J Loss Prev Process Ind. 2016;43:35–41.
Chiang CL, Liu SH, Lin YC, Shu CM. Thermal release hazard for the decomposition of cumene hydroperoxide in the presence of incompatibles using differential scanning calorimetry, thermal activity monitor III, and thermal imaging camera. J Therm Anal Calorim. 2017;127:1061–9.
Wu SH, Shyu ML, Yet-Pole I, Chi JH, Shu CM. Evaluation of runaway reaction for dicumyl peroxide in a batch reactor by DSC and VSP2. J Loss Prev Process Ind. 2009;22:721–7.
You ML, Liu MY, Wu SH, Chi JH, Shu CM. Thermal explosion and runaway reaction simulation of lauroyl peroxide by DSC tests. J Therm Anal Calorim. 2009;96:777–82.
Arkema, Arkema’s Crosby peroxides plant suffers hurricane-related damage. Addit Polym. 2017;7.
Chen WC, Shu CM. Prediction of thermal hazard for TBPTMH mixed with BPO through DSC and isoconversional kinetics analysis. J Therm Anal Calorim. 2016;126:1937–45.
Zaman F, Beezer AE, Mitchell JC, Clarkson Q, Elliot J, Davis AF, Willson RJ. The stability of benzoyl peroxide by isothermal microcalorimetry. Int J Pharm. 2001;227:133–7.
Kossoy AA, Akhmetshin YG. Simulation-based approach to design of inherently safer processes. Process Saf Environ Prot. 2012;90:349–56.
Kossoy AA, Belokhvostov VM, Koludarova EY. Thermal decomposition of AIBN: Part D: Verification of simulation method for SADT determination based on AIBN benchmark. Thermochim Acta. 2015;621:36–43.
Brown ME, Maciejewski M, Vyazovkin S, Nomen R, Sempere J, Burnham A, Opfermann J, Strey R, Anderson HL, Kemmler A, Keuleers R, Janssens J, Desseyn HO, Li CR, Tang TB, Roduit B, Malek J, Mitsuhashi T. Computational aspects of kinetic analysis: Part A: The ICTAC kinetics project-data, methods and results. Thermochim Acta. 2000;355:125–43.
Burnham AK. Computational aspects of kinetic analysis: Part D: The ICTAC kinetics project–multi thermal history model fitting methods and their relation to isoconversional methods. Thermochim Acta. 2000;355:165–70.
Dai X, Shi L, An Q, Qian W. Chemical kinetics method for evaluating the thermal stability of Organic Rankine Cycle working fluids. Appl Therm Eng. 2016;100:708–13.
Duh YS, Kao CS, Lee WLW. Chemical kinetics on thermal decompositions of dicumyl peroxide studied by calorimetry. J Therm Anal Calorim. 2017;127:1089–98.
Liu SH, Lin CP, Shu CM. Thermokinetic parameters and thermal hazard evaluation for three organic peroxides by DSC and TAM III. J Therm Anal Calorim. 2011;106:165–72.
Opfermann J. Kinetic analysis using multivariate non-linear regression. I. Basic concepts. J Therm Anal Calorim. 2000;2011(60):641–58.
Mettler TA8000, MT, Columbus, Ohio, USA, STARe software with solaris operating system. Operating Instructions, Switzerland. 1998.
Liu SH, Hou HY, Shu CM. Thermal hazard evaluation of the autocatalytic reaction of benzoyl peroxide using DSC and TAM III. Thermochim Acta. 2015;605:68–76.
Liu SH, Shu CM. Advanced technology of thermal decomposition for AMBN and ABVN by DSC and VSP2. J Therm Anal Calorim. 2015;121:533–40.
Martín-Lara M, Blázquez G, Zamora M, Calero M. Kinetic modeling of torrefaction of olive tree pruning. Appl Therm Eng. 2017;113:1410–8.
Kossoy AA, Akhmetshin YG. Identification of kinetic models for the assessment of reaction hazards. Process Saf Prog. 2007;26:209–20.
Kossoy AA, Sheinman IY. Comparative analysis of the methods for SADT determination. J Hazard Mater. 2007;142:626–38.
Kossoy AA, Benin AI, Akhmetshin YG. An advanced approach to reactivity rating. J Hazard Mater. 2005;118:9–17.
Andreozzi A, Buonomo B, Manca O. Numerical study of natural convection in vertical channels with adiabatic extensions downstream. Numer Heat Transf Part A. 2005;47:741–62.
Neylon MK, Savage PE. Analysis of non-isothermal heterogeneous autocatalytic reactions. Chem Eng Sci. 1996;51:851–8.
Parulekar SJ. Modal analysis and optimization of isothermal autocatalytic reactions. Chem Eng Sci. 1998;53:2379–94.
Kossoy AA, Hofelich TC. Methodology and software for assessing reactivity ratings of chemical systems. Process Saf Prog. 2003;22:235–40.
Beckmann JW, Wilkes JS, McGuire RR. 2,4,6-Trinitrotoluene thermal decomposition: kinetic parameters determined by the isothermal differential scanning calorimetry technique. Thermochim Acta. 1977;19:111–8.
Wang TS, Liu SH, Qian XM, You ML, Chou WL, Shu CM. Isothermal hazards evaluation of benzoyl peroxide mixed with benzoic acid via TAM III test. J Therm Anal Calorim. 2013;113:1625–31.
Huang CC, Peng JJ, Wu SH, Hou HY, You ML, Shu CM. Effects of cumene hydroperoxide on phenol and acetone manufacturing by DSC and VSP2. J Therm Anal Calorim. 2010;102:579–85.
ChemInform Saint-Petersburg (CISP), L., Thermal Safety Software. http://www.cisp.spb.ru, 2019.
Li XR, Koseki H. SADT prediction of autocatalytic material using isothermal calorimetry analysis. Thermochim Acta. 2005;431:113–6.
Lv J, Chen L, Chen W, Gao H, Peng M. Kinetic analysis and self-accelerating decomposition temperature (SADT) of dicumyl peroxide. Thermochim Acta. 2013;571:60–3.
Malow M, Wehrstedt KD. Prediction of the self-accelerating decomposition temperature (SADT) for liquid organic peroxides from differential scanning calorimetry (DSC) measurements. J Hazard Mater. 2005;120:21–4.
Gao PF, Liu SH, Zhang B, Cao CR, Shu CM. Complex thermal analysis and runaway reaction of 2,2′-azobis (isobutyronitrile) using DSC, STA, VSP2, and GC/MS. J Loss Prev Process Ind. 2019;60:87–95.
Liu SH, Cao CR, Lin WC, Shu CM. Experimental and numerical simulation study of the thermal hazards of four azo compounds. J Hazard Mater. 2019;365:164–77.
Acknowledgements
The authors are grateful to the financial support from Anhui Provincial Natural Science Foundation, China, under Contract Number 1908085ME125.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, SH., Wang, YR., Cao, CR. et al. Systematic process hazard assessment of three kinds of solid organic peroxides with kinetic analysis and heat transfer equilibrium. J Therm Anal Calorim 145, 451–466 (2021). https://doi.org/10.1007/s10973-020-09732-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09732-6