Abstract
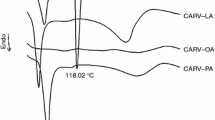
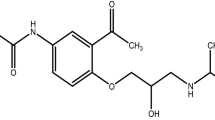
Carvedilol (CARVE) is an important cardiovascular drug with limited bioavailability. To improve its therapeutic performance, the investigation of new dosage forms is of great interest due its relevance in clinical applications. Therefore, the aim of this work was to evaluate the stability of CARVE and its drug–excipient compatibility to support its pharmaceutical development. Kinetic analysis under isothermal conditions using thermogravimetry was performed to determine the activation energy of CARVE through an Arrhenius plot. Differential scanning calorimetry, Fourier transform infrared spectroscopy, and optical microscopy were used to test binary mixtures of CARVE and selected excipients. The activation energy of CARVE was 81.2 kJ mol−1, and from the compatibility studies, all the excipients showed strong thermal interactions, presenting changes in the melting profile of the drug. In addition, analytical assays revealed no physical or chemical changes; because of this, all eight excipients studied are considered compatible and are recommended in formulations containing CARVE. All the evidence together attests to the low chemical reactivity of CARVE and provides useful information for the development of new pharmaceutical formulations containing CARVE.





Similar content being viewed by others
References
Sweetman SC. Martindale: the complete drug reference. 39th ed. London: Pharmaceutical Press; 2011.
Shim JB, Kim MJ, Kim SJ, Kang SJ, Lee JH, Kim HS, Lee D, Khang G. Dissolution properties of control released solid dispersion of carvedilol with HPMC and Eudragit RS. J Pharm Invest. 2012;42:285–91.
Loftsson T, Vogensen SB, Desbos C, Jansook P. Carvedilol: solubilization and cyclodextrin complexation: a technical note. AAPS PharmSciTech. 2008;9:425–30.
Bhosale P, Pore Y, Sayyad F. Preparation of amorphous carvedilol polymeric microparticles for improvement of physicochemical properties. J Pharm Invest. 2012;42:335–44.
Sharma A, Jain CP. Preparation and characterization of solid dispersions of carvedilol with PVP K30. Res Pharm Sci. 2010;5:49–56.
Saindane NS, Pagar KP, Vavia PR. Nanosuspension based in situ gelling nasal spray of carvedilol: development, in vitro and in vivo characterization. AAPS PharmSciTech. 2013;14:189–99.
Peres-Filho MJ, Gaeti MPN, Oliveira SR, Marreto RN, Lima EM. Thermoanalytical investigation of olanzapine compatibility with excipients used in solid oral dosage forms. J Therm Anal Calorim. 2011;104:255–60.
Shantikumar S, Sreekanth G, SurendraNath KV, JaferValli S, Satheeshkumar N. Compatibility study between sitagliptin and pharmaceutical excipients used in solid dosage forms. J Therm Anal Calorim. doi:10.1007/s10973-013-3329-3.
Sovizi MR, Hosseini SG. Studies on the thermal behavior and decomposition kinetic of drugs cetirizine and simvastatin. J Therm Anal Calorim. 2013;111:2143–8.
Tiţa B, Marian E, Fuliaş A, Jurca T, Tiţa D. Thermal stability of piroxicam. J Therm Anal Calorim. 2013;112:367–74.
Troy DB, Beringer P. Remington: the science and practice of pharmacy. 21st ed. Baltimore: Lippincott Williams & Wilkins; 2006.
Chen G, Ye J, Bao H, Yang P. Determination of the rate constants and activation energy of acetaminophen hydrolysis by capillary electrophoresis. J Pharm Biomed Anal. 2002;29:843–50.
Rowe RC, Sheskey PJ, Quinn ME. Handbook of pharmaceutical excipients. 6th ed. London: Pharmaceutical Press; 2009.
Maximiano FP, Novack KM, Bahia MT, Sa-Barreto LL, Cunha-Filho MSS. Polymorphic screen and drug–excipient compatibility studies of the antichagasic benznidazole. J Therm Anal Calorim. 2011;106:819–24.
Cunha-Filho MSS, Martínez-Pacheco R, Landín M. Compatibility of the antitumoral β-lapachone with different solid dosage forms excipients. J Pharm Biomed Anal. 2007;45:590–8.
Chaurasia G. Formulation and evaluation of cyclodextrins based carvedilol solid inclusion complexes by lyophilization method. Int J Pharm Bio Sci. 2013;4:612–20.
Silverstein RM, Francis X, Kiemle D. Spectrometric identification of organic compounds. 6th ed. Hoboken: Wiley; 1997.
Acknowledgements
The authors gratefully acknowledge Universidade Federal de Ouro Preto (UFOP) for their financial support. AT and MTB are recipients of productivity awards from CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talvani, A., Bahia, M.T., de Sá-Barreto, L.C.L. et al. Carvedilol: decomposition kinetics and compatibility with pharmaceutical excipients. J Therm Anal Calorim 115, 2501–2506 (2014). https://doi.org/10.1007/s10973-013-3491-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3491-7