Abstract
Nanosized powders as precursors have been synthesized via sol–gel technique to prepare ceramic composites in the LaPO4–Y2O3 and LaPO4–ZrO2 systems through normal sintering. Sol–gel technique was based on separate synthesis of LaPO4·nH2O, Y(OH)3, and ZrO(OH)2 components as sol using “reverse precipitation” (or “reverse flocculation”) technique, and further mixing them together to prepare (1‒x)LaPO4·nH2O–xY(OH)3 and (1‒x)LaPO4·nH2O–xZrO(OH)2 compositions as gels. During sol–gel synthesis, formation of hexagonal LaPO4·nH2O and Y(OH)3 or monoclinic ZrO(OH)2 was observed. As-prepared precursors of nanosized powders were then calcined at 850 °C for dehydration of the components and decomposition of yttrium and zirconium hydroxides to obtain nanosized (1‒x)LaPO4–xY2O3 and (1‒x)LaPO4–xZrO2 compositions, where x did not exceed 0.20 mole fraction. Ceramic composites were prepared by sintering these compositions subsequently at 1000, 1200, and 1300 °C for 24 h. Vickers microhardness was found to depend on x and sintering temperature. The influence of yttria and zirconia additions on dispersion of powders, their thermal behavior, specific surface area and ceramic fracture surface of the composites, and their open porosity was discussed.

Initial nanosized powders of 0.8LaPO4·nH2O−0.2Y(OH)3 (left) and 0.8LaPO4·nH2O−0.2ZrO(OH)2 (right) Fracture surfaces of 0.8LaPO4–0.2Y2O3 (left) and 0.8LaPO4–0.2ZrO2 (right) ceramic composites sintered at 1300 °C for 24 h.
Highlights
-
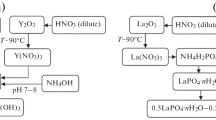
Sol–gel synthesis of nanopowders-precursors of (1‒x)LaPO4·nH2O–xY(OH)3 and (1‒x)LaPO4·nH2O–xZrO(OH)2 using separate and “inverse precipitation” of LaPO4·nH2O and Y(OH)3 or ZrO(OH)2 was performed.
-
Preparation of nanosized (1‒x)LaPO4–xY2O3 and (1‒x)LaPO4–xZrO2 compositions by preliminary calcining not compacting powders at 850 °C was carried out.
-
Sintering at 1000‒1300 °C to obtain (1‒x)LaPO4–xY2O3 and (1‒x)LaPO4–xZrO2 ceramic composites where mole fraction x ≤ 0.20 was performed.
-
Comparison of fracture surfaces of LaPO4, 0.8LaPO4–0.2Y2O3 and 0.8LaPO4–0.2ZrO2 ceramic samples sintered at 1000 and 1300 °C (24 h) was done.
-
Comparison of Vickers microhardness values for both systems was presented.
-
The influence of yttria and zirconia additions on dispersion of powders, their thermal behavior, specific surface area, and ceramic fracture surface was discussed.









Similar content being viewed by others
References
Mezentseva L, Osipov A, Ugolkov V, Kruchinina I, Popova V, Yakovlev A, Maslennikova T (2014) Solid solutions and thermal transformations in the nanosized LaPO4–YPO4–H2O and LaPO4–LuPO4–H2O systems. J Ceram Sci Tech 5:237–244
Mezentseva LP, Kruchinina I, Yu., Osipov AV, Kuchaeva SK, Ugolkov VL, Popova VF, Pugachev KE (2014) Nanopowders of orthophosphate LaPO4–YPO4–H2O system and ceramics based on them. Glass Phys Chem 40:356–361
Gavrichev KS, Ryumin MA, Tyurin AV, Khoroshilov AV, Mezentseva LP, Osipov AV, Ugolkov VL, Gusarov VV (2010) Thermal behavior of LaPO4·nH2O and NdPO4·nH2O nanopowders. //. J Therm Anal Calorim 102:809–811
Mezentseva LP, Kruchinina I, Yu., Osipov AV, Ugolkov VL, Popova VF, Lapenok A, Yu. (2015) The influence of the particularities of synthesis on the physicochemical properties of nanosized powders and ceramic samples of REE orthophosphates. Glass Phys Chem 41:668–671
Mezentseva LP, Kruchinina I, Yu., Osipov AV, Ugolkov VL, Popova VF, Kuchaeva SK (2017) Physical-chemical properties of nanopowders and ceramic samples of REE orthophosphates. Glass Phys Chem 43:98–105
Mezentseva LP, Osipov AV, Akatov AA, Doil’nitsin VA, Ugolkov VL, Popova VF, Maslennikova TP, Drozdova IA (2017) Chemical and thermal stability of phosphate ceramic matrices. Glass Phys Chem 43:83–90
Mezentseva LP, Osipov AV, Ugolkov VL, Akatov AA, Doilnitsyn VA, Maslennikova TP, Yakovlev AV (2018) Sol-gel synthesis, thermal behavior of nanopowders and chemical stability of La1–xHoxPO4 ceramic matrices. Glass Phys Chem 44:440–449
Mezentseva LP, Osipov AV, Ugolkov VL, Popova VF, Maslennikova TP, Kuchaeva SK, Yakovlev AV (2018) Physicochemical properties of nanosized powders of the LaPO4–DyPO4–H2O system. Glass Phys Chem 44:423–427
Wang R, Pan W, Chen J, Fang M, Cao Z, Luo Y (2003) Synthesis and sintering of LaPO4 powder and its application. Mater Chem Phys 79:30–36
Min W, Miyahara D, Yokoi K, Yamaguchi T, Daimon K, Hikichi Y, Matsubara T, Ota T (2001) Thermal and mechanical properties of sintered LaPO4–Al2O3 composites. Mater Res Bull 36:939–945
Sasidharan S, Komban R, Nambiar S, Nair BN, Padmanabhan M, Warrier KG, Hareesh US (2017) In: Pillai SC, Hehir S (eds). Sol-gel materials for energy, environment and electronic applications. Springer Int Publ. Chapter 10. Sol-gel lanthanum phosphate: A versatile ceramic material for diverse functional applications. p 285–312
Balamurugan K, Uthayakumar M, Sankar S, Hareesh US, Warrier KGK (2018) Modeling and surface texturing on surface roughness in machining LaPO4–Y2O3 composite. Mater Manuf Process 33:405–413
Ren X, Zhao M, Pan W (2014) Thermal conductivity and mechanical properties of YSZ/LaPO4 composites. J Mater Sci 49:2243–2251
Li Z, Liu J, Li S, Du H (2009) Microstructure, mechanical properties and thermal shock resistance of ZrO2–LaPO4composites. J Alloys Comps 480:863–866
Sankar S, Raj AN, Jyothi CK, Warrier KGK, Padmanabhan PVA (2012) Room temperature synthesis of high temperature stable lanthanum phosphate–yttria nano composite. Mater Res Bull 47:1835–1837
Balamurugan K, Uthayakumar M, Sankar S, Hareesh US, Warrier KGK (2018) Effect of abrasive waterjet machining on LaPO4/Y2O3 ceramic matrix composite. J AustrCeram Soc 54:205–214
Kim S-H, Sekino T, Kusunose T, Hirvonen AT (2007) Thermal properties and microstructure of zirconia/monazite-type LaPO4 composites for powder preparation methods. Mater Sci Forum 544‒545:909–912
Shijina K, Sankar S, Midhun M, Firozkhan M, Nair BN, Warrier KG, Hareesh UNS (2016) Very low thermal conductivity in lanthanum phosphate–zirconia ceramic nanocomposites processed using a precipitation–peptization synthetic approach. New J Chem 40:5333–5337
Min W, Daimon K, Matsubara T, Hikichi Y (2002) Thermal and mechanical properties of sintered machinable LaPO4–ZrO2 composites. Mater Res Bull 37:1107–1115
Zhang C, Fei J, Guo L, Yu J, Zhang B, Yan Z, Ye F (2018) Thermal cycling and hot corrosion behavior of a novel LaPO4/YSZ double-ceramic-layer thermal barrier coating. Ceram Int 44:8818–8826
Sankar S, Warrier KG (2011) Aqueous sol–gel synthesis of lanthanum phosphate nano rods starting from lanthanum chloride precursor. J Sol-Gel Sci Technol 58:195–200
Bregiroux D, Lucas S, Champion E, Audubert F, Bernache-Assollant D (2006) Sintering and microstructure of rare earth phosphate ceramics REPO4 with RE=La, Ce or Y. J Europ. Ceram Soc 26:279–287
Lucas S, Champion E, Bregiroux D, Bernache-Assollant D, Audubert F (2004) Rare earth phosphate powders RePO4·nH2O (Re=La, Ce or Y)–Part I. Synthesis and characterization. J Solid State Chem 177:1302–1311
Diaz-Guillén JA, Fuentes AF, Gallini S, Colomer MT (2007) A rapid method to obtain nanometric particles of rhabdophane LaPO4·nH2O by mechanical milling. J Alloys Compd 427:87–93
Bregiroux D, Lucas S, Champion E, Audubert F, Bernache-Assollant D (2006) Sintering and microstructure of rare earth phosphate ceramics REPO4 with RE=La, Ce or Y. J Europ. Ceram Soc 26:279–287
Petrunin VF, Popov VV, Hongzhi Z, Timofeev AA (2004) Synthesis of nanocrystalline high-temperature zirconia phases. Inorg Mater 40:251–258
Onoda H, Nariai H, Moriwaki A, Maki H, Motooka I (2002) Formation and catalytic characterization of various rare earth phosphates. J Mater Chem 12:1754–1760
Acknowledgements
We gratefully acknowledge the financial support of this work by the Russian Foundation for Basic Research, project no. 18-03-00488-a.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mezentseva, L., Osipov, A., Ugolkov, V. et al. Sol–gel synthesis of precursors and preparation of ceramic composites based on LaPO4 with Y2O3 and ZrO2 additions. J Sol-Gel Sci Technol 92, 427–441 (2019). https://doi.org/10.1007/s10971-019-05003-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05003-5