Abstract
Impregnation of tri-n-octylamine (TOA) into Siplite LX-16 resin, by two commonly used methods namely; wet and dry impregnation method, was studied and applied for uranium extraction from sulfate medium. The resin beads were characterized using FT-IR and FE-SEM analysis. The influence of different parameters such as effect of TOA concentration, pH, aqueous sample and eluant flow rates were investigated. Maximum sorption of U(VI) was obtained using 30% TOA/Siplite LX-16 prepared by the dry method at a pH range of 1.4–1.6. Flow rate of 1.0 mL/min for the pregnant sample was favorable for quantitative sorption of uranium during the extraction step while the flow rate of 0.5 mL/min for eluant solution was effective for the elution step.
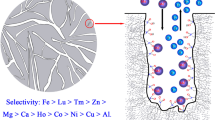
Graphic abstract
TOA-impregnated Siplite LX-16 resin has been properly prepared and applied for U(VI) extraction from sulfuric acid medium.
















Similar content being viewed by others
References
Fouad H, Abu Elenein S, Orabi A, Abdulmoteleb S (2019) A new extractant impregnated resin for separation of traces of uranium and thorium followed by their spectrophotometric determination in some geological samples. SN Appl Sci 1:309–326
Kabay N, Cortina JL, Trochimczuk A, Streat M (2010) Solvent impregnated resins (SIRs)—methods of preparation and their applications. React Funct Polym 70(8):484–496
Warshawsky A (1981) Extraction with solvent-impregnated resins. In: Marinsky JA, Marcus Y (ed) Ion exchange and solvent extraction: a series of advances, vol 8, 1st edn. Marcel-Dekker, New York
Amaral JCBS, Morais CA (2010) Thorium and uranium extraction from rare earth elements in monazite sulfuric acid liquor through solvent extraction. Miner Eng 23(6):498–503
Ramadevi G, Sreenivas T, Navale AS, Padmanabhan NPH (2012) Solvent extraction of uranium from lean grade acidic sulfate leach liquor with Alamine 336 reagent. J Radioanal Nucl Chem 294(1):13–18
Daher AM, Abdel Wanees S, Kellah HMA, Ali AH (2014) Removal of uranium from sulfate leach liquor of salcrete deposits using tri-n-octyl amine. J Radioanal Nucl Chem 299:493–499
Hellé G, Mariet C, Cote G (2015) Liquid-liquid extraction of uranium(VI) with Aliquats 336 from HCl media in microfluidic devices: combination of micro-unit operations and online ICP-MS determination. Talanta 139:123–131
Rao TP, Metilda P, Gladis JM (2006) Preconcentration technique for uranium(VI) and uranium(IV) prior to analytical determination-an overview. Talanta 68(4):1047–1064
Semnani F, Asadi Z, Samadfam M, Sepehrian H (2012) Uranium(VI) sorption behavior onto amberlite CG-400 anion exchange resin: effects of pH, contact time, temperature and presence of phosphate. Ann Nucl Energy 48:21–24
Reinoso-Maset E, Ly J (2016) Study of uranium(VI) and radium(II) sorption at trace level on kaolinite using a multisite ion exchange model. J Environ Radioact 157:136–148
Li JQ, Gong LL, Feng XF, Zhang L, Wu HQ, Yan CS, Xiong YY, Gao HY, Luo F (2017) Direct extraction of U(VI) from alkaline solution and seawater via anion exchange by metal–organic framework. Chem Eng J 316:154–159
Cortina JL, Miralles N, Aguilar M, Sastre AM (1994) Solvent impregnated resins containing di(2-ethylhexyl)phosphoric acid. I. Preparation and study of the retention and distribution of the extractant on the resin. Solvent Extr Ion Exc 12(2):349–369
Cortina JL, Miralles N, Aguilar M, Sastre AM (1994) Solvent impregnated resins containing di(2-Ethylhexyl) phosphoric acid. II. Study of the distribution equilibria of Zn, Cu and Cd. Solvent Extr Ion Exc 12(2):371–391
Juang RS (1999) Preparation, properties and sorption behavior of impregnated resins containing acidic organophosphorus extractants. Proc Natl Sci Counc ROC(A) 23(3):353–364
Rovira M, Hurtado L, Cortina JL, Arnaldos J, Sastr AM (1998) Recovery of palladium(II) from hydrochloric acid solutions using impregnated resins containing Alamine 336. React Funct Polym 38(2–3):279–287
Muraviev D, Ghantous L, Valiente M (1998) Stabilization of solvent-impregnated resin capacities by different techniques. React Funct Polym 38(2–3):259–268
Mendoza RN, Medina TIS, Vera A, Rodríguez MA, Guibal E (2000) Study of the sorption of Cr(III) with XAD-2 resin impregnated with di-(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272). Solvent Extr Ion Exc 18(2):319–343
Jerabek K, Hankova L, Strickovsky AG, Warshawsky A (1996) Solvent impregnated resins: relation between impregnation process and polymer support morphology I. Di(2ethylhexyl) dithiophosphoric acid. React Funct Polym 28(2):201–207
Singh KK, Pathak SK, Kumar M, Mahtele AK, Tripathi SC, Bajaj PN (2013) Study of uranium sorption using D2EHPA-impregnated polymeric beads. J Appl Polym Sci 130(5):3355–3364
Matsunaga H, Ismail AA, Wakui Y, Yokoyama T (2001) Extraction of rare earth elements with 2-ethylhexyl hydrogen 2-ethylhexyl phosphonate impregnated resins having different morphology and reagent content. React Funct Polym 49(3):189–195
Karve M, Pandey K (2012) Sorption studies of U(VI) on Amberlite XAD-2 resin impregnated with Cyanex272. J Radioanal Nucl Chem 293(3):783–787
Abbasi WA, Streat M (1998) Sorption of uranium from nitric acid solution using TBP-impregnated activated carbons. Solvent Extr Ion Exc 16(5):1303–1320
Khatab AF, Sheta ME, Mahfouz MG, Tolba AA (2007) Studies on the sorption behaviours of Th(IV) and U(VI) from aqueous sulphate solutions using Impregnated resin. Isotop Rad Res 39(2):291–305
Sato T (1972) The extraction of uranium(VI) from hydrochloric acid solutions by long-chain alkyl quaternary ammonium chloride. J Inorg Nucl Chem 34(12):3835–3843
Zhu Z, Pranolo Y, Cheng CY (2013) Uranium solvent extraction and separation from vanadium in alkaline solutions. Sep Sci Technol 48:1402–1408
Crane P, Virnig M, Bender J, Macknzie M, Dudley K (2010) Analysis and troubleshooting in uranium solvent extraction circuits. In: Proceedings of ALTA uranium conference, Perth
Zhu Z, Cheng CY (2011) A review of uranium solvent extraction: its present status and future trends. In: Proceedings of ALTA uranium conference, Perth
Eberle AR, Lerner MW, Coldbeck CG, Rodden CJ (1970) Titrimetric determination of uranium in product, fuel and scrap materials after ferrous iron reduction in phosphoric acid. US Atomic Energy Commission, New Brunswick Laboratory, NBL-252, NJ
Helaly OS, Abd El-Ghany MS, Moustafa MI, Abuzaid AH, Abd El-Monem NM, Ismail IM (2012) Extraction of cerium(IV) using tributyl phosphate impregnated resin from nitric acid medium. Trans Nonferrous Met Soc China 22:206–214
Merdivan M, Duz MZ, Hamamci C (2001) Sorption behaviour of uranium(VI) with N, N-dibutyl-Nʹ-benzoylthiourea impregnated in Amberlite XAD-16. Talanta 55(3):639–645
Seyhan S, Merdivan M, Demirel N (2008) Use of o-phenylene dioxydiacetic acid impregnated in Amberlite XAD resin for separation and preconcentration of uranium(VI) and thorium(IV). J Hazard Mater 152(1):79–84
Wojcik G, Hubicki Z, Ryczkowski J (2009) Investigations of chromium(III) and (VI) ions sorption on SIR by using photoacoustic and DRS methods. Acta Phys Pol A 116(3):432–434
Karve M, Pandey K (2010) Cyanex272 impregnated on Amberlite XAD-2 for separation and preconcentration of U(VI) from uranmicrolite (leachates) ore tailings. J Radioanal Nucl Chem 285(3):627–633
Plasil J, Kasatkin A, Skoda R, Novak M, Kallistova A, Dusek M, Skala R, Fejfarová K, Cejka J, Meisser N, Goethals H, Machovic V, Lapcak L (2013) Leydetite, Fe(UO2)(SO4)2(H2O)11, a new uranyl sulfate mineral from Mas d’Alary, Lodève, France. Mineral Mag 77(4):429–441
Olds TA, Plasil J, Kampf AR, Burns PC, Nash BP, Marty J, Rose TP, Carlson SM (2018) Redcanyonite, (NH4)2Mn[(UO2)4O4(SO4)2](H2O)4, a new zippeite-group mineral from the Blue Lizard mine, San Juan County, Utah, USA. Mineral Mag 82(6):1261–1275
Arden TV, Humphries R, Lewis JA (1958) The separation of uranium from other metals in sulphate solution by fractional hydrolysis. II. Precipitation in the presence of phosphate, arsenate and silicate. J Appl Chem 8(3):151–159
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mosleh, M.A., El-Hakim, E.H., Ahmed, A.Z. et al. Tri-n-octylamine impregnated Seplite LX-16 resin for extraction of U(VI) from sulfate medium. J Radioanal Nucl Chem 323, 909–919 (2020). https://doi.org/10.1007/s10967-019-07010-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-07010-w