Abstract
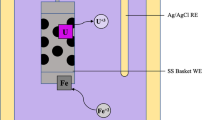
The electro-redox behavior of uranium(III) on Mo electrode in NaCl–KCl molten salt in the temperature range 973–1073 K has been investigated using cyclic voltammetry electrochemical method and so on, such research will help to understand uranium behavior in pyro-reprocessing. The results showed that UCl3 could be reduced into uranium metal in a quasi-reversible one-step process exchanging three electrons. The diffusion coefficients of U(III) ions were determined and the activation energy for diffusion was found to be 55.794 kJ mol−1. The apparent standard potentials of U(III)/U(0) at several temperatures were calculated. The thermodynamic properties of UCl3 have also been investigated.










Similar content being viewed by others
References
Nuclear Energy Agency (2000) Pyrochemical separations, pp 14–16
Shirai O (2006) Electrochemical behavior of actinides and actinide nitrides in LiCl–KCl eutectic melts. J Alloy Compd 408:1267–1273
Nuclear Energy Agency (2004) Pyrochemical separations in nuclear applications, pp 60–66
Nagarajan K (2011) Development of pyrochemical reprocessing for spent metal fuels. Asian Nucl Prospect 7:431–436
Reddy B (2004) Electrochemical studies on the redox mechanism of uranium chloride in molten LiCl–KCl eutectic. Electrochem Acta. 49:2471–2478
Bottomley D (2005) Electrochemistry of uranium in molten LiCl–KCl eutectic. J Electrochem Soc 152:1109–1115
Kim G (2012) A study on the electrochemical deposition behavior of uranium ion in a LiCl–KCl molten salt on solid and liquid electrode. J Electroanal Chem 682:128–135
Wang C (2013) Electrochemical separation of uranium and cerium in molten LiCl–KCl. J Radioanal Nucl Chem 298:581–586
Vladimir A (2010) On the formation of uranium(V) species in alkali chloride melts. Pure Appl Chem 82:1710–1717
Li S (2002) Anodic process of electrorefining spent nuclear fuel in molten LICL–KCL–UCL3/CD system. Meet Electrochem Soc 201:1–13
Jia YH (2015) Pyrex membrance Ag/AgCl reference electrode used in molten salt. Inorg Chem Ind. 47:60–63
Jia YH (2015) Performance of boron mitride membrance Ag/AgCl reference electrode used in molten salt. Inorg Chem Ind. 47:67–71
Wang YQ (2015) Progress of Ag/AgCl reference electrode in high-temprature chloride molten salt. Mod Chem Ind. 35:21–25
Martinot L (1975) Thermodynamic properties of dilute solution of ThCl4 in (Li-K)Cl and (Na-K)Cl eutectics. J Inorg Nucl Chem 37:315–319
Michael D (2004) An approach to optimised calculations of the potential energy surfaces for the case of electron transfer reactions at a metal/solution interface. Chem Phys Lett 399:307–314
Renat R (2005) Microscopic modelling of the reduction of a Zn(II) aqua-complex on metal electrodes. Chem Phys 310:257–268
Srinivasan R (1964) Electrode potentials of thorium tetrachloride in alkali chloride melts. Can J Chem 42:1315–1322
Delahay P (1954) New instrumental methods in electrochemistry. Interscience Publishers, New York
Cassayre L (2008) On the formation of U–Al alloys in the molten LiCl–KCl eutectic. J Electrochem Mater. 1:79–85
Kuznetsov S (2005) Electrochemical behavior and some thermodynamic properties of UCl4 and UCl3 dissolved in a LiCl–KCl eutectic melt. J Electrochem Soc 4:203–212
Tang H (2014) Electrochemical behavior of LaCl3 and morphology of La deposit on molybdenum substrate in molten LiCl-KCl eutectic salt. Electrochim Acta 119:120–130
Zhang JS (2014) Electrochemistry of actinides and fission products in molten salts-data review. J Nucl Mater 447:271–284
Acknowledgements
Many appreciate to the support of The National Natural Science Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
YanHong, J., Hui, H., Hui, C. et al. Electrochemical behavior of uranium(III) in NaCl–KCl molten salt. J Radioanal Nucl Chem 311, 1763–1770 (2017). https://doi.org/10.1007/s10967-016-5131-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-5131-8