Abstract
Thin films of polyvinyl alcohol (PVA) loaded with various concentrations of (f-MWCNTs) were prepared via the regular casting method. The physicochemical characteristics of pure and PVA/f-MWCNTs films were investigated with various techniques; X-ray diffractometer (XRD), Fourier transform infrared (FTIR), X-ray photoelectron spectroscopy (XPS), UV-visible spectroscopy (UV-Vis), and thermogravimetric analysis (TGA). Moreover, the impact of loading ratios of f-MWCNTs on the dielectric constant and AC conductivity of the PVA/f-MWCNTs nanocomposite film was examined. XRD results suggest a possible improvement in the crystallinity of the PVA/f-MWCNTs composite film compared to a pristine one. Also, FTIR and XPS results confirm the formation of a hydrogen bond between PVA chains and f-MWCNTs. The optical band gaps of the PVA/f-MWCNTs composite film decrease, while the optical conductivity (σopt), dielectric constant (έ), and AC conductivity increase with increasing wt% of f-MWCNTs in the PVA matrix. Further, the thermal stability of PVA/f-MWCNTs films improved in comparison with that of PVA film.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, the industrial and scientific societies have smitten many considerations for the fabrication of polymer-based nanocomposites due to their distinctive characteristics, like ease of synthesis, low cost, light weight, enhancement in thermal, optical, and optoelectronic characteristics, and multifaceted and multi-functional capabilities [1]. Moreover, the polymer nanocomposites are used in dielectric materials to develop capacitors with enhanced refractive index and dielectric permittivity, and optoelectronic and optical devices. Among the synthetic organic polymers, polyvinyl alcohol (PVA) has been widely employed for the production of nanocomposite materials because of its easy processability, high hydrophilic and thermoplastic nature, optical transparency, good optical characteristics, high dielectric abilities, high charge storage capacity, and outstanding film formation capability [2,3,4]. Besides, its solubility in an aqueous solution makes the dispersion of the introduced nanofiller in the solvent easy, and as a result, the prepared nanocomposite is almost nontoxic [3].
Multiwall Carbon nanotubes (MWCNTs) have attracted the interest of numerous researchers because of their excellent thermal stability as well as their mechanical, optical, electric, structural, and chemical characteristics [5,6,7]. They can be utilized in various applications, like chemical sensing [8], medication delivery, and Nano-electronics [9]. Despite their excellent properties, the outer wall of MWCNTs is chemically inert, which limits their use in industrial applications [10]. Therefore, the outer surface of MWCNTs needs to be functionalized to make them accepted for various applications. The surface functionalization of MWCNTs is the introduction of different functional groups, like carboxyl (-COOH), amine (-NH), and hydroxyl (-OH) onto the MWCNTs surface. The various catalytic and physicochemical properties of the modified MWCNTs surface are caused by these new functional groups. The functionalization process of the outer surfaces of MWCNTs can be carried out via various techniques such as mechanical, chemical, electrochemical, and plasma processing technology [11, 12]. The most favourable technique is the application of plasma processing technology, which is free from chemical waste and residuals and is a nondestructive and quick method [13,14,15]. Therefore, in the present work, MWCNTs are functionalized by applying plasma processing technology for the sake of good dispersion and compatibility with the PVA matrix.
According to the literature, some of the papers regarding the functionalization of CNTs and their loading ratio impact on physicochemical properties of polymer nanocomposites are listed here; Banerjee et al. [16] prepared and characterized chemically functionalized amorphous CNTs/PVA composites. They found that the inclusion of f-CNTs into PVA matrix improve the crystallinity, optical, thermal and electrical properties of the film. Maghfirah et al. [17] functionalized the pristine MWCNT by the oxidation method using a mixture of H2SO4 and HNO3 and investigated the impact of f-MWCNTs concentration on the chemical, optical, and mechanical properties of PVA film. Meikhail et al. [18] reported an enhancement in the structural, optical, and mechanical characteristics of a Poly (vinyl alcohol)/Sodium alginate) blend film by loading the oxidized MWCNTs. Zidan et al. [10] investigate the effect of the chemically functionalized MWCNTs on the structure and optical properties of a PVP/PVA blend film. They confirmed homogenous dispersion of MWCNTs in the fabricated film and an improvement in the optical properties of the samples. Mahmood and Habubi [19] tuned the mechanical and electrical properties of PVP/PVA blend film with CNTs by varying the contents of CNTs in the film. Ahmad et al. [1] studied the impact of MWCNTs on the structural, optical, and electrical properties of (PVA/PEO) blend film. It was found that the inclusion of MWCNTs into the film improved its electrical conductivity, and the crystallinity as well as optical properties.
In this work, we examined the effects of incorporation of plasma-functionalized MWCNTs with various loading ratios (0.001%, 0.002, 0.004, and 0.01 wt%) on the structure and other physical properties of PVA matrix. Our results confirms the efficient functionalization of the MWCNTs improves their dispersion in the PVA matrix as well as improves the optical, thermal and electrical characteristics of the fabricated films.
Experimental setup
Materials
The MWCNTs (~ 30 nm in diameter) from Hanwha Nanotech, South Korea and PVA grains (M.W = 470,00 g/mol) from Sigma-Aldrich were used to fabricate PVA/f-MWCNTs films. The deionized (DI) water, from Al-Gomhoria Company, Cairo, Egypt, was used as a solvent for PVA grains.
Methods
MWCNTs functionalization
The plasma treatment of the MWCNTs were performed using RF dielectric barrier discharge at treatment conditions: argon/nitrogen mixture plasma, treatment time 6 min at discharge conditions of 60 W and 500 Pa [15]. The creation of polar groups on surface of plasma-treated MWCNTs (f-MWCNTs) is expect decreases the inherited aggregation characters between adjacent MWCNTs, hence improving their dispersion and compatibility with the PVA matrix.
Film preparation
The pristine PVA and PVA filled with various wt% percentages of f-MWCNTs were prepared with traditional casting technique [12]. In brief, 1 g of PVA dissolved in 50 mL of DI water under continuous stirring for one day until getting a transparent solution. Then, the plasma-f-MWCNTs with the desired concentration (wt% = 0.001, 0.002, 0.004, and 0.01) were weighted and added to PVA solution and stirred for 60 min followed by sonication for other 30 min to get a uniform dispersion of f-MWCNTs in PVA solution. Finally, the homogeneous mixtures solution of PVA/f-MWCNTs were casted on a glass plates and kept in air for 24 h to dry. The thickness of the produced films ranged from 30 to 60 μm as measured with digital micrometer.
Film characterizations
The pristine PVA and PVA/f-MWCNTs films were examined with various techniques. X-ray diffraction spectrometer (Rigaku International Corp., Japan) with λ = 1.543 Å to examine structure and crystanality of the films. The chemical bonding/compositions of the prepared films were evaluated using FTIR (Nicolet™ iS™ 5 FTIR Spectrometer, USA) and XPS (Thermo Scientific™ K Alpha spectrometer) using Al K alpha (1486.6 eV) with flood gun for charge compensation. C 1s at 284.5 eV was used as standard calibration. The optical properties were evaluated using UV-VIS (UV-5200, M&A Instruments Inc, China) spectrophotometer. The thermal characteristics were analyzed using Thermo Gravimetric (TGA, NETZSCH instruments, Deutschland) in Nitrogen environment with heating rate of 10 °C per minute. The TGA analysis done with Netzsch proteus 70 software. The electrical characteristics of the prepared films, were investigated using AC impedance spectroscopy methods, frequency range 300 Hz to 3 GHz at room temperature.
Results and discussion
X-ray diffraction (XRD)
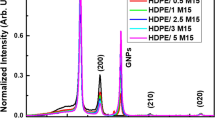
Figure 1 displays the XRD patterns of f-MWCNTs, PVA, and PVA + 0.01 wt% f-MWCNTs nanocomposite films. The XRD of f-MWCNT shows a broad peak at 2θ = 26.93◦ and another tiny peak at 2θ = 42◦ that corresponds to the (002) and (100) plans of graphite structure, respectively [20, 21]. The XRD of pristine PVA displays a broad diffraction peak at 2θ = 20◦ reflecting the semi-crystalline nature of the pure PVA [4]. After incorporating f-MWCNTs in the PVA matrix, the PVA characteristic peak at 2θ = 20◦ becomes more intense, suggesting a possible improvement in the crystallinity of PVA/f-MWCNTs composite films [1]. This behavior is probably due to the doping effect of f-MWCNTs which enhance delocalization of PVA chain. Zhang et al. [22] attributed such behavior to the possible re-ordering of PVA chains along f-MWCNTs. Further, a tiny peak can be seen at 2θ = 26o presumably reflects the existing of f-MWCNT in the PVA/f-MWCNTs composite films. The low-intensity of the graphite characteristic peak (002) in the XRD pattern of PVA/f-MWCNTs film could be due to homogenous dispersion of plasma-f-MWCNTs in the PVA matrix [10]. The lattice strain (ε), is calculated by using the FWHM (β) and Bragg angle (θ) values from the XRD results (according to the equation ε = β/4tanθ) [23]. The (ε) was 0.0242 for both pure PVA and PVA/0.01 wt% f-MWCNTs films, indicating good interaction and no lattice mismatch between the PVA matrix and the loaded plasma f-MWCNTs.
Fourier transform infrared analysis (FTIR)
Figure 2 displays FTIR spectra of pristine PVA and PVA with varying loading ratios of plasma-functionalized MWCNTs films. Figure 2a displays the characteristic bands of pristine PVA and they can be identified as follows: a shallow broad band at ~ 3000–3550 cm− 1 indexed to hydroxyl group (O-H) of PVA [24, 25], weak band at 2900–2950 cm− 1 that corresponds to C-H bond of the PVA chain [26]. The bands in the range 1300–1450 cm− 1, at ~ 1096 cm− 1, and 844 cm− 1 are assigned to C-H in -CH2, C-O (carbonyl group) and C-C bond of the PVA backbone, respectively. The f-MWCNTs spectrum shown in Fig. 2b revealed peaks in the range 2800–2950 assigned to -C-H group, weak peak at 1743 cm− 1 assigned to C = O vibration that formed as a result of the oxidation of some carbon atoms of MWCNTs side surface during plasma treatment/and or when it exposed to ambient air after treatment [27]. Also, one can notice tiny bands at 1631 cm− 1 and 1100 cm− 1 that ascribed to C = N imines and C-N/C-O group, respectively. The FTIR spectra of PVA/f-MWCNTS (Fig. 2c, d) preserve the same characteristic peaks of pristine PVA, however, a remarkable improve in peak intensities/broadening have been observed. Furthermore, when the PVA matrix is loaded with f-MWCNTS, the frequency of the (O-H), C-H in -CH2, and C-O peaks of the PVA is slightly shifted to a higher wavenumber. These findings suggest a good intermolecular interaction and establish hydrogen bonding between the hydroxyl groups of the PVA matrix and the formed polar groups (C = O, C = N and C-N) on the f-MWCNTs surface.
XPS analysis
The chemical interaction between PVA and plasma-functionalized MWCNTs was investigated further using XPS analysis. Figure 3a depicts the survey spectra of pristine PVA and PVA/f-MWCNTs composite films. As evident, the survey spectra of pristine PVA polymer have strong peaks at 284.5 eV and at 532.2 eV that assigned for carbon C 1s and Oxygen O 1s peaks, respectively. The intensity of C 1s and O 1s peaks of PVA increased upon the incorporation of f-MWCNTs (0.004%wt) in the PVA matrix composite. Further, one can observe a tiny peak at 400 eV that is assigned to nitrogen N 1s confirming the successful functionalization of MWCNTs. The ratio of O/C in PVA sample was 0.158 and it is increased to 0.24 in PVA/f-MWCNT composite sample. The increment of O/C ratio implies an increase in the polar groups along polymer chains or at the PVA/f-MWCNTs surface. To explore the type of carbon bonding and estimate their relative intensities, high-resolution spectra C 1s have been measured and plotted in Fig. 3b, c. As clear, the C 1s of a pure PVA sample can be deconvolved into 3 sup-peaks characteristics of the PVA polymer [28] such as; C-CH bond at 283.82 eV, C-OH (methylene carbon singly bonded to OH group) at 285.2 eV, and O–C = O species at 287.3 eV, as displayed in Fig. 3b. The presence of the O–C = O bond in the XPS spectra of PVA probably a moieties of methyl acetate group in the pristine PVA sample [29].
The high-resolution spectra C 1s of PVA/f-MCNT shows the same sub-peaks, however, with varying intensities. In particular, the peak intensities assigned to C-OH and O–C = O species increased upon the incorporation of f-MWCNT. We note that the sub-peak assigned for the bond O–C = O shifted to higher BE for PVA/f-MCNT sample, suggesting this sub-peak might composed of O–C = O and O = C–N bonds. These results are in agreement with FTIR data shown in Fig. 2, and confirms the interaction between PVA and the incorporated f-MWCNTs. Further, the band head of the sub peaks of the PVA/f-MWCNT sample shifts to higher binding energy. This is probably due to the improved conductivity of the PVA upon incorporation of f-MWCNTs, as well as, the formation of O = C-N (288.3 eV) species in the PVA/f-MWCNT sample.
Optical properties analysis
The UV-visible absorption spectra of pristine PVA and PVA/f-MWCNTs nanocomposite films with varying wt% of f- MWCNTs are illustrated in Fig. 4. The absorption spectrum of pristine PVA has a sharp absorption edge in the UV range and absorption dramatically decreases in the visible range. The absorption edge at λ = 195 nm of pristine PVA, reflects its semi-crystalline nature that is consistent with the XRD results. The absorption of PVA/f-MWCNTs over the entire examined UV-Vis range increased with the rising loading ratio of f-MWCNTs in the PVA matrix, that can be attributed to the high-absorption property of MWCNTs. Furthermore, the absorption edge of the PVA/f-MWCNTs films shifted to a higher wavelength 250 nm, implying the reduction of the optical energy gap values as the wt (%) of f- MWCNTs in PVA matrix increased [30]. Our findings are consistent with those previously reported for PVA loaded with ZnO [31], Fe [4] and Fe2O3 [32]. The high absorption (low transmittance) of PVA/MWCNTs films makes them convenient for different applications such as solar collectors and UV shielding [32, 33].
In the present work, the optical band gab Eg was determined from the experimental data of the absorbance, [A (λ)], of PVA and PVA/f-MWCNTs films using the ineffective thickness method (ITM) via the following equation [34]:
where D is a constant, E is photon energy and m is an exponent, which characterizes the transition process and takes values 2 and 0.5 for indirect and direct allowed transitions, respectively.
Figure 5 displays a plot of [A (λ) E]0.5 and [A (λ) E]2 versus E for indirect and direct permitted transition, respectively. The Eg values of PVA and PVA/f-MWCNTs films were obtained directly from the interception of the extrapolation of the straight parts of the [A(λ)E]0.5 – E and [A(λ)E]2– E curves to the axis of energy at zero [A(λ)E]0.5 and [A(λ)E]2, respectively and the results are summarized in Table 1. As inferred from Table 1, the indirect and direct Eg values of PVA decrease with the increasing loading (wt %) of f-MWCNTs in the PVA matrix. For pure PVA, indirect and direct Eg values are 4.532 and 5.802 eV, which decrease to 3.388 and 4.919 eV for PVA/f-MWCNTs films with a loading ratio of 0.01%. The reduction of Eg with increasing wt% of f-MWCNTs could be ascribed to the creation of localized states in the forbidden band gap caused by Van Hove singularities [12], as well as increase the creation of polarons. The reported Eg values of the examined samples are in good agreement with previous studies [10, 12, 30, 35].
The extinction coefficient (k = αλ/4π) of the PVA and PVA/f-MWCNTs nanocomposite films were determined to estimate their refractive index (n). The reflectance R(λ) of the investigated samples was obtained from the experimental results of the transmittance T(λ) and optical absorbance A(λ) spectra by the following Eq. [4]:
The refractive index was estimated using the equation below [4]:
Figure 6 shows the extinction coefficient (k) and the refractive index (n) versus the wavelength for PVA and PVA/f-MWCNTs films. Both (k and n) clearly rise with the rise in plasma-functionalized MWCNTs nanoparticles concentration in PVA matrix. The figure also demonstrates that the PVA and PVA/f-MWCNTs films have a lower refractive index in the visible than UV range of the spectrum. The refractive index of PVA film at λ = 700 nm increases from 2.46 to 3.4 with increasing f-MWCNTs wt% from 0 to 0.01 wt%. Ahmed et al. [1] reported an increment in the refractive index of PEO/PVA film from 1.5 to 1.7 with increasing MWCNTs wt% from 0 to 4 wt% at the same wavelength. The enhancement in the k values is attributed to the rise in the PVA film’s optical absorption by addition of f-MWCNT. Meanwhile, the increase in the values of (n) could be attributed to the rise in density of packing as the f-MWCNTs wt% in the PVA matrix increases, as well as the structural modification in PVA matrix after introducing plasma-functionalized MWCNTs nanoparticles in PVA films [36]. The high refractive index of PVA films after filling with plasma-functionalized MWCNTs nanoparticles makes them suitable for the fields of reflective–antireflective coatings, lenses, filters and opto-electric applications [37].
Another important optical parameter of a material is the optical conductivity σopt, which is the material’s reply to the charge carriers diffusion induced by the incident photon’s energy [38, 39]. The optical conductivity of the synthesized PVA/f-MWCNTs nanocomposite films was computed using equation [40]:
where α optical absorption coefficient and c speed of light. Figure 7 displays the optical conductivity (σopt) dependence of photon energy E for the pure PVA and PVA/f-MWCNTs nanocomposite samples. As clear, σopt increased with increasing photon energy as well as the loading wt% of f-MWCNTs. Further, at the relatively low photo energy of E < 3 eV, electrons receive less energy to be excited, hence the optical conductivity is slightly increased. As the photon energy increased > 3 eV, the electrons received enough energy to transient from the (V.B) to the (C.B) of the PVA films; leading to increase the concentration of charge carriers and rapid increase of optical conductivity values of the PVA film.
The improved σopt values with increasing wt% of f-MWCNTs in PVA matrix, are attributed to the formation of additional energy levels in the forbidden band gap, which tends to facilitate the electrons transport from a state to another [38]. Therefore, the Eg decreased and σopt increased. Our results are consistent with those reported in the literature [30]. The high optical conductivity σopt values for PVA/f-MWCNTs nanocomposite films support the application in the fields of information and computing processing, photoelectric and optoelectronic devices.
Dielectric and electrical characterization
The dielectric constant έ and ac conductivity σac of PVA and PVA/f-MWCNTs samples have been calculated using the measured data of dielectric properties by the following equations [41]:
where t is the film thickness, ɛ0 is the permittivity of free space, Z is the sample impedance, A and CP are the cross-section area and the capacitance of the sample, respectively. Figure 8a depicts the dielectric constant (έ) dependence on frequency at room temperature for PVA and PVA/f-MWCNTs nanocomposite samples.
From the figure it can be observed that for PVA and PVA/f-MWCNTs nanocomposite films, έ takes a decreasing trend as the applied frequency increases. This decrease can be ascribed to the rise of polarization, which was a result of contributions from various types of polarization in the final polarization [42]. The first polarization type was polarization by charge carriers at the electrodes that took place at low frequencies. The second type is bipolar polarization, which occurs when π-electric moments molecules varying their orientations along the applied electric field. In addition to the ionic polarization which resulted from the movement of positive ions and negative charges relative to one another. The last type is the electrical polarization which resulted from the valence electrons displacement from their stable location round the positively charged nucleus. Because of the occurring of ionically and electrically polarization longer than the preceding two kinds, polarization decreases with rising the frequency. As a result the obtained decrease in the values of έ for PVA and PVA/f-MWCNTs nanocomposite samples with the increasing in frequency. Further, increasing the load wt% of f-MWCNTs in the PVA matrix increases the έ value; at f = 1 kHz, the pure PVA has έ =3.2 and it increases to 4.6 when doping 0.01 wt% f-MWCNTs in the PVA matrix. This increment in the value of έ is more than that reported for chemically functionalized MWCNTs/PVA composites, where the έ values at the same frequency were 0.25 and 0.75 for PVA and 1 wt% MWCNTs/ PVA, respectively [43]. The increment in the value of έ with increasing the load wt% of f-MWCNTs in PVA matrix is attributed to the dipolar interaction of the f-MWCNTs with PVA matrix. The behaviour of (έ) of PVA and PVA/f-MWCNTs samples versus the applied frequency and f-MWCNTs concentration is similar to that previously reported for PVC/f-MWCNTs films [12].
The ac conductivity (σac) versus frequency at room temperature for PVA and PVA/f-MWCNTs nanocomposite samples is presented in Fig. 8b. The ac conductivity is increased as the wt% of f-MWCNTs in PVA matrix is increased. At frequency 5 MHz, the σac of pristine PVA film is 5.32 µS/cm while it is 7.89 µS/cm for PVA/0.01 wt% f-MWCNTs film at the same frequency. This indicates that loading PVA with 0.01 wt% f-MWCNTs enhances σac of the PVA film by about 50% in comparsion to that of pristine PVA film. This finding is more than that reported for PVA/MCNTs compsites, where σac was increased from 4.23 × 10− 8 S/cm to 6.1 × 10− 8 S/cm as the concentration of MCNTs in PVA matrix increases from 0 to 40%(v/v) [17]. The increase in σac for PVA/MWCNTs sample probably suggests that the f-MWCNTs, which is conducting filler, forms conducting network on the insulating PVA matrix [44].
Thermal analysis
Thermogravimetric Analysis (TGA) is an effective technique for evaluating the thermal degradation and heat resistance of the polymer nanocomposite materials. The impact of introducing f-MWCNTs on the thermal stability of PVA film was examined via thermogravimetric analysis. Figure 9 depicts the TGA and its derivative DTA for pure PVA and PVA + 0.01 wt% f-MWCNTs nanocomposite films.
It was seen that the profile of Weight loss of pure PVA and PVA + 0.01wt% f-MWCNTs nanocomposite films was similar in the whole examined temperature range. The decomposition of PVA and PVA + 0.01wt% f-MWCNTs nanocomposite films is a multistage decomposition process, resulting in multistage weight loss under the nitrogen atmosphere. The first stage takes place at temperatures ranging from 25 to 180 ∘C. The slight weight loss in this stage is attributed to the dehydration of PVA chains, volatilization of moisture, and the solvent residuals. The next stage is between 200 and 300 ∘C, a remarkable loss in weight was noticed for both PVA and PVA + 0.01 wt% f-MWCNTs nanocomposite films. This remarkable weight loss results from the breakdown of PVA macromolecular chains into minute fragments [45]. The tertiary stage is located at temperatures ranging from 400 to 520 ∘C, in which the Weight loss is less than that in the second stage and is due to the thermal decomposition of the main chains in the PVA matrix [46]. The inclusion of plasma-functionalized MWCNTs into the PVA matrix improved the thermal resistance of PVA slightly, shifting the degradation temperature to slightly higher values. Lin et al. [47] reported the shifting of the initial decomposition temperature of PVA film from 222 to 238 ∘C by loading PVA matrix with 0.25 wt% of MWCNTs. The DTA for pure PVA and PVA + 0.01wt% MWCNTs samples is shown in Fig. 9b, it can be noticed that maximum weight loss occurs at temperatures of 252 and 255 ∘C for PVA and PVA + 0.01wt% MWCNTs nanocomposite films, respectively. Abdel-Fattah et al. [12] reported the shifting of the temperature at maximum weight loss from 283.6 to 300.4 when PVC matrix is loaded with 0.02 wt% of MWCNTs. Hence, the introduction of plasma-functionalized MWCNTs in a PVA polymer matrix at different concentrations (0.001, 0.002, 0.004, and 0.01 wt%) enhanced the thermal stability characteristics of the PVA films slightly.
Conclusion
The XRD results showed an improvement in the crystallinity of PVA/f-MWCNTs films with increasing the wt% of f-MWCNTs in the film. FTIR and XPS analysis confirm the creation of the hydrogen bonding between PVA chains and f-MWCNT that is an indication for the homogenous distribution of f-MWCNTs in PVA matrix. With increasing the wt% of f-MWCNTs, the values of optical energy gaps decreased, while extinction coefficient (k), refractive index (n), optical conductivity (σopt) and the dielectric constant (έ) values were improved. Further, increasing the percentage of f-MWCNTs in the PVA matrix improved also the AC conductivity and thermal stability of PVA. These improvements in the physicochemical properties of PVA film with loading f-MWCNTs are suitable for potential use in industrial applications.
References
Ahmad AA, Al-Bataineh QM, Bani-Salameh AA, Telfah AD (2022) Optical, optoelectronic, structural, and chemical characterizations of (PEO-PVA)/MWCNT nanocomposite films. Surf Interfaces 33:102202
Ghobadi S, Sadighikia S, Papila M, Cebeci F, Gürsel SA (2015) Graphene- reinforced poly (vinyl alcohol) electrospun fibers as building blocks for high performance nanocomposites. RSC Adv 5:85009
Khan MM, Pal S, Hoque MM, Alam MR, Younus M, Kobayashi H (2019) Simple fabrication of PVA – ZnS composite films with superior photocatalytic performance: enhanced luminescence property, morphology, and thermal stability. ACS Omega 4:6144
Soliman TS, Vshivkov SA (2019) Effect of Fe nanoparticles on the structure and optical properties of polyvinyl alcohol nanocomposite films. J Non-Cryst Solids 519:119452
Che Y, Chen H, Gui H, Liu J, Liu B, Zhou C (2014) Review of carbon nanotube nanoelectronics and macroelectronics. Semicond Sci Technol 29:073001
Kumar S, Nehra M, Kedia D, Dilbaghi N, Tankeshwar K, Kim KH (2018) Carbon nanotubes: a potential material for energy conversion and storage. Prog Energy Combust Sci 64:219
Sun L, Wang X, Wang Y, Zhang Q (2017) Roles of carbon nanotubes in novel energy storage devices. Carbon 122:462
Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, Dai H (2000) Nanotube molecular wires as chemical sensors. Sci 287:622
Zhang W, Zhang Z, Zhang Y (2011) Nanoscale Res Lett 6:555
Zidan HM, Abdelrazek EM, Abdelghany AM, Tarabiah AE (2019) Characterization and some physical studies of PVA/PVP filled with MWCNTs. J Mater Res Technol 8:904
Tomova A, Gentile G, Grozdanov A, Errico ME, Paunović P, Avella M, Dimitrov AT (2016) Functionalization and characterization of MWCNT produced by different methods. Acta Phys Pol A 129:405
Abdel–Fattah E, Alharthi AI, Fahmy T (2019) Spectroscopic, optical and thermal characterization of polyvinyl chloride–based plasma–functionalized MWCNTs composite thin films. Appl Phys A: Mater Sci Process 125:475
Abdel-Fattah E, Ogawa D, Nakamura K (2017) Oxygen functionalization of MWCNTs in RF-dielectric barrier discharge Ar/O2 plasma. J Phys D Appl Phys 50:265301
Hosseini H, Ghaffarzadeh M (2022) Surface functionalization of carbon nanotubes via plasma discharge: a review. Inorg Chem Commun 138:109276
Abdel-Fattah E, Ogawa D, Nakamura K (2020) Nitrogen functionalization of MWCNTs in Ar-N2 dielectric barrier discharge–gas ratio effect. Mater Sci Eng B 261:114680
Banerjee D, Jha A (2012) Chattopadhyay, synthesis and characterization of water soluble functionalized amorphous carbon nanotube-Poly(vinyl alcohol) composite. Macromol Res 20:1028
Maghfirah A, Yudianti R, Fauzi P, Sinuhaji LG, Hutabarat (2018) Preparation of Poly(Vinyl) alcohol–multiwalled carbon nanotubes nanocomposite as conductive and transparent film using casting method. J Phys Conf Ser 1116:032017. https://doi.org/10.1088/1742-6596/1116/3/032017
Meikhail MS, Hezma AM (2018) Mohamed, characterization and physical studies of poly (vinyl alcohol)/Sodium alginate/ multiwall carbon nanotube biocomposites. J Text Color Polym Sci 15:95
Mahmood HS, Habubi NF (2022) Physical properties of PVA:PVP blend reinforced by MWCNT. J Phys Conf Ser 2322:012068. https://doi.org/10.1088/1742-6596/2322/1/012068
Luo F, Ma C, Tang Y, Zhou L, Ding Y, Chen G (2022) Sandwich-structured flexible PVA/CS@ MWCNTs composite films with high thermal conductivity and excellent electrical insulation. Polymers 14:2512
Singh C, Srivastava S, Ali MA, Gupta TK, Sumana G, Srivastava A, Mathur RB, Malhotra BD (2013) Carboxylated multiwalled carbon nanotubes based biosensor for aflatoxin detection. Sens Actuators B Chem 185:258
Zhang X, Zhang J, Liu Z (2005) Tubular composite of doped polyaniline with multi-walled carbon nanotubes. Appl Phys A 80:1813
Rahmah MI, Sabry RS, Aziz WJ (2022) Preparation and antibacterial activity of superhydrophobic modifed ZnO/PVC nanocomposite. J Bio Engin 19:139
Aslam M, Kalyar MA, Raza ZA (2016) Synthesis and structural characterization of separate graphene oxide and reduced graphene oxide nanosheets. Mater Res Express 3:105036
Yuan X (2011) Enhanced interfacial interaction for effective reinforcement of poly(vinyl alcohol) nanocomposites at low loading of graphene. Polym Bull 67:1785
Aslam M, Kalyar MA, Raza ZA (2018) Investigation of zinc oxide-loaded poly(vinyl alcohol)nanocomposite films in tailoring their structural, optical and mechanical properties. J Electron Mater 47:3912
Yan YH, Cui J, Chan-Park MB, Wang X, Wu QW (2007) Systematic studies of covalent functionalization of carbon nanotubes via argon plasma-assisted UV grafting. Nanotechnology 18:115712
Beamson G, Briggs D (1992) High resolution XPS of organic polymers. Wiley, New York
Sreedhar B, Chattopadhyay DK, Sri Hari Karunakar M, Sastry ARK (2006) Thermal and surface characterization of plasticized starch polyvinyl alcohol blends crosslinked with epichlorohydrin. J Appl Poly Sci 101:25
Helal AI, Vshivkov SA, Zaki MF, Elkalashy ShI, Soliman TS (2021) Effect of carbon nano tube in the structural and physical properties of polyvinyl chloride films. Phys Scr 96:085804
Atta A, Abdel Reheem AM, Abdeltwab E (2020) Ion beam irradiation effects on surface morphology and optical properties of ZnO/PVA composites. Surf Rev Lett 27:1950214
Donya H, Taha TA, Alruwaili A, Tomsah IBI (2020) Ibrahim, micro-structure and optical spectroscopy of PVA/iron oxide polymer nanocomposites. J Mater Res Technol 9(4):9189
Ariyawiriyanan W, Meekaew T, Yamphang M, Tuenpusa P, Boonwan J, Euaphantasate N, Muangchareon P, Chungpaibulpatana S (2013) Thermal efficiency of solar collector made from thermoplastics. Energy Procedia 34:500
Ghobadi N (2016) Derivation of ineffective thikness method for investigating of the exact behavior of the optical transitions in nanostructured thin films. Mater Sci: Mater Electron 27:8951
Abdullah ET, Hasan SM, Naje AN (2013) Optical properties of PVC-MWCNT nano composites. Indian J Pure Appl Phys 51:77
Singh R, Gaur MS, Tiwari RK (2015) J Electrostat 76:95
Aziz SB, Ahmed HM, Hussein AM, Fathulla AB, Wsw RM, Hussein RT (2015) Tuning the absorption of ultraviolet spectra and optical parameters of aluminum doped PVA based solid polymer composites. J Mater Sci Mater Electron 26:8022
Taha TA, Saleh A (2018) Dynamic mechanical and optical characterization of PVC/fGO polymer nanocomposites. Appl Phys A: Mater Sci Process 124:600
Krishnan P, Gayathri K, Gunasekaran S, Anbalagan G (2014) Optical, spectral and thermal properties of organic nonlinear optical single crystal: 2,3-Dimethoxy-10-oxostrychnidinium hydrogen oxalate dihydrate. Optik (Stuttg) 125:3852
Abutalib MM, Yahia IS (2019) Analysis of the linear/nonlinear optical properties of basic fuchsin dye/FTO films: controlling the laser power of red/green lasers. Optik (Stuttg) 179:145
Nasrallah DA, El-Sayed NM, Farag OF (2021) Modification of hydrophilic, optical and electrical properties of bisphenol-A based polycarbonate polymeric films using DC O2 plasma J. Polym Res 28:380
Abdullah SH, Salman MO, Humud HR (2019) Electrical properties of copper iodine prepared by exploding wire. Karbala Int J Mod Sci 5:146
Alghunaim NS (2016) Optimization and spectroscopic studies on carbon nanotubes/PVA nanocomposites. Results in Physics 6:456
Jose T, George SC, Maya MG, Thomas S (2015) Functionalized MWCNT and PVA nanocomposite membranes for dielectric and pervaporation applications. J Chem Eng Process Technol 6:233
Zuo G, Liu X, Fahlman M, Kemerink M (2018) High seebeck coefficient in mixtures of conjugated polymers. Adv Funct Mater 28:1703280
Alzaharani HAH (2022) CuO and MWCNTs nanoparticles filled PVA–PVP nanocomposites: morphological, optical, thermal, dielectric, and electrical characteristics. J Inorg Organomet Polym Mater 32:1913
Lin JH, Lin Z, Pan Y, Hsieh C, Huang C, Lou C (2016) Thermoplastic polyvinyl alcohol/multiwalled carbon nanotube composites: Preparation, mechanical properties, thermal properties, and electromagnetic shielding effectiveness. J Appl Polym Sci 43474:1
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farag, O.F., Abdel‑Fattah, E. Synthesis and characterization PVA/plasma-functionalized MWCNTs nanocomposites films. J Polym Res 30, 183 (2023). https://doi.org/10.1007/s10965-023-03550-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03550-8