Abstract
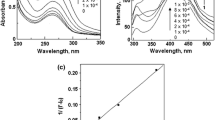
The host–guest interaction of tolmetin (TOL) with β-cyclodextrin (β-CD) and the influence of human serum albumin (HSA) on the formation of the inclusion complex were studied by 1D and 2D NMR spectroscopy. The TOL/β-CD inclusion complex formed at a molar ratio of 1:1 with a binding constant value of 2164.5 L·mol−1. Data analysis showed that the addition of 10 μmol·L−1 of HSA weakened the strength of TOL binding to β-CD (K a = 1493 L·mol−1). The interaction of TOL with HSA in the absence and presence of β-CD was studied by analyzing the fluorescence quenching data. The Stern–Volmer quenching constants and the binding constants are found to be smaller in the presence of β-CD, suggesting that β-CD hinders the strong interaction of TOL with HSA by complex formation. Additionally, the presence of β-CD does not induce conformational and microenvironmental changes on HSA.










Similar content being viewed by others
References
Carter, D.C., Ho, J.X.: Structure of serum albumin. Adv. Protein Chem. 45, 153–203 (1994)
Sudha, N., Enoch, I.V.M.V.: Interaction of curculigosides and their β-cyclodextrin complexes with bovine serum albumin: a fluorescence spectroscopic study. J. Solution Chem. 40, 1755–1768 (2011)
Natesan, S., Sowrirajan, C., Dhanaraj, P., Enoch, I.V.M.V.: Capping of silybin with cyclodextrin influences its binding with bovine serum albumin. A study of fluorescence spectroscopy and molecular modeling. Bull. Korean Chem. Soc. 35, 2114–2122 (2014)
Sowrirajan, C., Natesan, S., Dhanaraj, P., Enoch, I.V.M.V.: Binding of a chromen-4-one Schiff’s base with bovine serum albumin: capping with β-cyclodextrin influences the binding. J. Biomol. Struct. Dyn. 33, 1945–1956 (2015)
Natesan, S., Sowrirajan, C., Yousuf, S., Enoch, I.V.M.V.: Mode of encapsulation of Linezolid by β–cyclodextrin and its role in bovine serum albumin binding. Carbohydr. Polym. 115, 589–597 (2015)
Yan, J., Wu, D., Ma, X., Wang, L., Xu, K., Li, H.: Spectral and molecular modeling studies on the influence of β-cyclodextrin and its derivatives on aripiprazole–human serum albumin binding. Carbohydr. Polym. 131, 65–74 (2015)
Redondo, J., Capdevila, A.: Latorre,: I.: Host-guest complexation of omeprazole, pantoprazole and rabeprazole sodium salts with cyclodextrins: an NMR study on solution structures and enantiodiscrimination power. J. Incl. Phenom. Macrocycl. Chem. 73, 225–236 (2012)
Floare, C.G., Pirnau, A., Bogdan, M.: 1H NMR spectroscopic characterization of inclusion complexes of tolfenamic and flufenamic acids with β-cyclodextrin. J. Mol. Struct. 1044, 72–78 (2013)
Medronho, B., Valente, A.J.M., Costa, P.: Inclusion complexes of rosmarinic acid and cyclodextrins: stoichiometry, association constants, and antioxidant potential. Colloid Polym. Sci. 292, 885–894 (2014)
Pirnau, A., Floare, C.G., Bogdan, M.: The complexation of flurbiprofen with β-cyclodextrin: a NMR study in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 78, 113–120 (2014)
Martinez-Alonso, A., Losada-Barreiro, S., Bravo-Diaz, C.: Encapsulation and solubilization of the antioxidants gallic acid and ethyl, propyl and butyl gallate with β-cyclodextrin. J. Mol. Liq. 210, 143–150 (2015)
Neamtu, S., Mic, M., Bogdan, M., Turcu, I.: The artifactual nature of stavudine binding to human serum albumin. A fluorescence quenching and isothermal titration calorimetry study. J. Pharm. Biomed. Anal. 72, 2134–2138 (2013)
Djedaïne, F., Lin, S.Z., Perly, B., Wouessidjewe, D.: High-field nuclear magnetic resonance techniques for the investigation of a β-cyclodextrin:indomethacin inclusion complex. J. Pharm. Sci. 79, 643–646 (1990)
Bogdan, M., Caira, M.R., Farcas, S.I.: Inclusion of the niflumic acid anion in β-cyclodextrin: A solution NMR and X-ray structural investigation. Supramol. Chem. 14, 427–435 (2002)
Floare, C.G., Bogdan, M.: CONSTEQ—a program for association constants determination using solution NMR data. AIP Conf. Proc. 1565, 48–52 (2013)
Junquera, E., Mendicuti, F., Aicart, E.: Driving forces for the inclusion of the drug tolmetin by β-cyclodextrin in aqueous medium. Conductometric and molecular modeling studies. Langmuir 15, 4472–4479 (1999)
Neamtu, S., Tosa, N., Bogdan, M.: Spectroscopic investigation of tolmetin interaction with human serum albumin. J. Pharm. Biomed. Anal. 85, 277–282 (2013)
van de Weert, M., Stella, L.: Fluorescence quenching and ligand binding: a critical discussion of a popular methodology. J. Mol. Struct. 993, 144–150 (2011)
Acknowledgements
This work was financially supported by UEFISCDI Romania, Project PCE-2011-3-0032.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bogdan, M., Floare, C.G., Pirnau, A. et al. Competitive Binding of Tolmetin to β-Cyclodextrin and Human Serum Albumin: 1H NMR and Fluorescence Spectroscopy Studies. J Solution Chem 46, 44–57 (2017). https://doi.org/10.1007/s10953-016-0549-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-016-0549-8