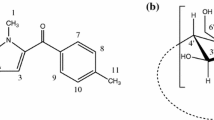
Abstract
We report, in this paper, the interaction of cabergoline in the free form and β-cyclodextrin-bound form with bovine serum albumin. The stoichiometry and the binding constant of the Cabergoline–β-cyclodextrin inclusion complex are reported based on UV–Vis absorption and fluorescence spectroscopic studies and the structure of the 1:1 inclusion complex is proposed using two-dimensional rotating-frame Overhauser effect spectroscopy. Molecular docking is used to propose the mode of interaction of cabergoline with bovine serum albumin. The apparent binding constants for the interaction of cabergoline with bovine serum albumin in water and in aqueous β-cyclodextrin solution are compared. The average distance between donor and acceptor is altered in the presence of β-cyclodextrin, as indicated by Förster resonance energy transfer. The influence of β-CD on the binding of the small molecule with bovine serum albumin is discussed.





Similar content being viewed by others
References
Reichmann, H.: Long-term treatment with dopamine agonists in idiopathic Parkinson’s disease. J. Neurol. 247, 17–19 (2000)
Webster, J., Piscitelli, G., Polli, A., Ferrari, C.I., Ismail, I., Scanlon, M.F.: The cabergoline comparative study group: a comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. N. Eng. J. Med. 331, 904–909 (2004)
Abs, R., Verhelst, J., Maiter, D., Van Acker, K., Nobels, F., Coolens, J.L., Mahler, C., Beckers, A.: Cabergoline in the treatment of acromegaly: a study in 64 patients. J. Clin. Endocrinol. Metabol. 833, 74–78 (1998)
Petrossians, P., Ronci, N., Valdes-Socin, H., Kalife, A., Stevenaert, A., Bloch, B., Tabarin, A., Beckers, A.: ACTH silent adenoma shrinking under cabergoline. Eur. J.Endocrinol. 144, 51–57 (2001)
Annamaria, C., Giovanni, V., Paolo, C., Francesco, B., Antonio, C., Michele, D.R., Stefano, Z., Gaetano, L.: Outcome of cabergoline treatment in men with prolactinoma: effects of a 24-month treatment on prolactin levels, tumor mass, recovery of pituitary function, and semen analysis. J. Clin. Endocrinol. Metabol. 89, 1704–1711 (2004)
Zucconi, M., Oldani, A., Castronovo, C., Ferini-Strambi, I.: Cabergoline is an effective single-drug treatment for restless legs syndrome: clinical and actigraphic evaluation. Sleep 26, 815–818 (2003)
Cozzi, R., Attanasio, R., Barausse, M., Dallabonzana, D., Orlandi, P., Re, N.D., Branca, V., Oppizzi, G., Gelli, D.: Cabergoline in acromegaly: a renewed role for dopamine agonist treatment ? Eur. J. Endocrinol. 139, 516–521 (1998)
Colmenarejo, G.: In silico prediction of drug binding strengths to human serum albumin. Med. Res. Rev. 23, 275–301 (2003)
Sugio, S., Kashima, A., Mochizuki, S., Noda, M., Kobayashi, K.: Crystal structure of human serum albumin at 2.5 Å resolution. Protein Eng. 12, 439–446 (1999)
Molla, A., Vasavanonda, S., Kumar, G., Sham, H.L., Johnson, M., Grabowski, B., Denissen, J.F., Kohlbrenner, W., Plattner, J.J., Leonard, J.M., Norbeck, D.W., Kempf, D.J.: Human serum attenuates the activity of protease inhibitors toward wild-type and mutant human deficiency virus. Virology 250, 255–262 (1998)
Rang, H.P., Dale, M.M., Ritter, J.: Molecular Pharmacology, 3rd edn. Churchill Livingstone, New York (1995)
Riihimaki, L.H., Vainio, M.J., Heikura, J.M.S., Valkonen, K.H., Virtanen, V.T., Vuorela, P.M.: Binding of phenolic compounds and their derivatives to bovine and reindeer β-lactoglobulin. J. Agric. Food Chem. 56, 7721–7729 (2008)
Xiao, J.B., Cao, H., Wang, Y.F., Zhao, J.Y., Wei, X.L.: Glycosylation of dietary flavonoids decreases the affinities for plasma protein. J. Agric. Food Chem. 57, 6642–6648 (2009)
He, X.M., Carter, D.C.: Atomic structure and chemistry of human serum albumin. Nature 358, 209–215 (1992)
Papadopoulou, A., Green, R.J., Frazier, R.A.: Interaction of flavonoids with bovine serum albumin: a fluorescence quenching. J. Agric. Food Chem. 53, 158–163 (2005)
Turnidge, J.: Pharmacokinetics and pharmacodynamic of fluoroquinolones. Drugs 58, 29–36 (1999)
Sinjan, C., Nand, K.: Unraveling the energetics and mode of the recognition of antibiotics tetracycline and rolitetracycline by bovine serum albumin. Chem. Biol. Drug Des. 80, 693–705 (2012)
Maliwal, B.P., Rao, A.G.A., Rao, M.S.N.: Spectroscopic study of the interaction of gossypol with bovine serum albumin. Int. J. Pept. Protein Res. 25, 382–388 (1985)
Meier-Kriesche, H.U., Shaw, L.M., Korecka, M., Kaplan, B.: Pharmacokinetics of mycophenolic acid in renal insufficiency. Ther. Drug Monit. 22, 27–30 (2000)
Kanakis, C.D., Tarantilis, P.A., Polissiou, M.G., Diamantoglou, S., Tajmir-Riahi, H.A.: Antioxidant flavonoids bind human serum albumin. J. Mol. Struct. 798, 69–74 (2006)
Tang, J.H., Luan, F., Chen, X.G.: Binding analysis of glycyrrhetinic acid to human serum albumin: fluorescence spectroscopy, FT-IR, and molecular modeling. Bioorg. Med. Chem. 14, 3210–3217 (2006)
Valero, M., Carrillo, C.: Effect of binary and ternary poly-ethylene glycol and/or β-cyclodextrin complexes on the photochemical and photosensitizing properties of naproxen. J. Photochem. Photobiol. B 74, 151–160 (2004)
Zhang, G., Shuang, S., Dong, C., Liu, D., Choi, M.M.F.: Investigation on DNA assembly to neutral red-cyclodextrin complex by molecular spectroscopy. J. Photochem. Photobiol. B 74, 127–134 (2004)
Szejtli, J.: Cyclodextrin Technology. Kluwer, Dordrecht (1998)
Pitha, J., Milecki, J., Fales, H., Pannell, L., Uekama, K.: Hydroxypropyl-β-cyclodextrin: preparation and characterization; effects on solubility of drugs. Int. J. Pharm. 29, 73–82 (1986)
Brewster, M., Hora, M., Simpkins, J., Bodor, N.: Uses of 2-hydroxpropyl-β-cyclodextrin as a solubilizing and stabilizing excipient for protein drugs. Pharm. Res. 8, 792–795 (1991)
Heindell, N.D., Egolf, R.A., Stefely, J.S.: Effect of liposome and cyclodextrin entrapment on retardation of glutathione decomposition of nitroimidazolylsulfones. J. Pharm. Sci. 79, 862–865 (1990)
Brewster, M., Loftsson, T., Eastes, K., Lin, J.L., Fridriksdottir, H., Bodor, N.: Effect of various cyclodextrins on solution stability and dissolution rate of doxorubicin hydrochloride. Int. J. Pharm. 79, 289–299 (1992)
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 85, 1017–1025 (1996)
Rajewski, R.A., Stella, V.J.: Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J. Pharm. Sci. 85, 1142–1169 (1996)
Atwood, J.L., Davies, J.E., MacNicol, D.D., Vogtle, F., Lehn, J.-M.: Comprehensive Supramolecular Chemistry. Elsevier, New York (1996)
Enoch, I.V.M.V., Swaminathan, M.: Flourimetric and prototropic studies on the inclusion complexation of 2-amino and 4-aminodiphenyl ethers with β-cyclodextrin: unusual behavior of 4-aminodiphenyl ether. J. Lumin. 127, 713–720 (2007)
Enoch, M.V., Rajamohan, R., Swaminathan, M.: Fluorimetric and prototropic studies on the inclusion complexation of 3,3′-diaminodiphenylsulphone with β-cyclodextrin and its unusual behavior. Spectrochim. Acta A77, 473–477 (2010)
Enoch, I.V.M.V., Swaminathan, M.: Fluorimetric study on molecular recognition of β-cyclodextrin with 2-amino-9-fluorenone. J. Fluoresc. 16, 501–510 (2006)
Sideris, E.E., Koupparis, M.A., Macheras, P.E.: Effect of cyclodextrins on protein binding of drugs: the diflunisal/hydroxypropyl-β-cyclodextrin model case. Pharm. Res. 11, 90–95 (1994)
Sudha, N., Enoch, I.V.M.V.: Binding of curculigosides and their β-cyclodextrin inclusion complexes with bovine serum albumin: A fluorescence spectroscopic study. J. Solution Chem. 40, 1755–1768 (2011)
Sameena, Y., Enoch, I.V.M.V.: Spectroscopic investigation of interaction of 6-methoxyflavanonoe and its β-cyclodextrin inclusion complex with calf thymus DNA. Chem. Pap. 66, 787–794 (2012)
Lakowicz, J.R.: Principles of Fluorescence Spectroscopy, 3rd edn, pp. 277–284. Springer, New York (2006)
Loftsson, T., Mason, M.: Cyclodextrins in topical drug formulations: theory and practice. Int. J. Pharm. 225, 15–30 (2001)
Chandrasekaran, S., Sameena, Y., Enoch, I.V.M.V., Santhanam, V.: Binding of the host-guest complex of 7-aminoflavone/β-cyclodextrin with calf thymus DNA: a spectroscopic and molecular docking study. J. Solution Chem. 43, 1132–1146 (2014)
Chandrasekaran, S., Sameena, Y., Enoch, I.V.M.V.: Tuning the binding of Coumarin 6 with DNA by molecular encapsulators: effect of β-cyclodextrin and C-hexylpyrogallol[4]arene. J. Mol. Recognit. 27, 640–652 (2014)
Förster, T.: Delocalized excitation and excitation transfer. In: Sinanoglu, O. (ed.) Modern Quantum Theory. Academic Press, New York (1996)
Chandrasekaran, S., Sameena, Y., Enoch, I.V.M.V.: The unusual fluorescence quenching of Coumarin 314 by β-cyclodextrin and the effect of β-cyclodextrin on its binding with calf thymus DNA. Aust. J. Chem. 67, 256–265 (2014)
Valero, M., Esteban, B., Peláez, R., Rodríguez, L.J.: Naproxen: hydroxypropyl-β-cyclodextrin: polyvinylpyrrolidone ternary complex formation. J. Incl. Phenom. Macro. Chem. 48, 157–163 (2004)
Acknowledgments
We express our sense of gratitude to the SAIF, Indian Institute of Technology–Madras, Chennai, for the assistance in NMR measurements. We thank the DST–SERB for the Project SR/FT/CS-062/2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sudha, N., Enoch, I.V.M.V. Binding Modes of Cabergoline to Bovine Serum Albumin in Free- and β-Cyclodextrin-Encapsulated Forms: Differences in Quenching Behavior and Förster Resonance Energy Transfer. J Solution Chem 44, 1367–1381 (2015). https://doi.org/10.1007/s10953-015-0355-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0355-8