Abstract
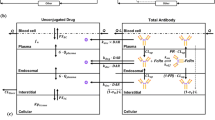
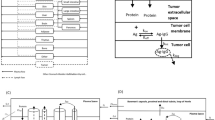
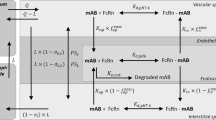
The main objective of this manuscript was to validate the ability of the monoclonal antibody physiologically-based pharmacokinetic (PBPK) model to predict tissue concentrations of antibodies in the human. To accomplish this goal, preclinical and clinical tissue distribution and positron emission tomography imaging data generated using zirconium-89 (89Zr) labeled antibodies were obtained from the literature. First, our previously published translational PBPK model for antibodies was expanded to describe the whole-body biodistribution of 89Zr labeled antibody and the free 89Zr, as well as residualization of free 89Zr. Subsequently, the model was optimized using mouse biodistribution data, where it was observed that free 89Zr mainly residualizes in the bone and the extent of antibody distribution in certain tissues (e.g., liver and spleen) may be altered by labeling with 89Zr. The mouse PBPK model was scaled to rat, monkey, and human by simply changing the physiological parameters, and a priori simulations performed by the model were compared with the observed PK data. It was found that model predicted antibody PK in majority of the tissues in all the species superimposed over the observed data, and the model was also able to predict the PK of antibody in human tissues reasonably well. As such, the work presented here provides unprecedented evaluation of the antibody PPBK model for its ability to predict tissue PK of antibodies in the clinic. This model can be used for preclinical-to-clinical translation of antibodies and for prediction of antibody concentrations at the site-of-action in the clinic.







Similar content being viewed by others
References
Covell DG, Barbet J, Holton OD, Black CD, Parker R, Weinstein JN (1986) Pharmacokinetics of monoclonal immunoglobulin G1, F (ab′) 2, and Fab′ in mice. Can Res 46(8):3969–3978
Baxter LT, Zhu H, Mackensen DG, Jain RK (1994) Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Can Res 54(6):1517–1528
Ferl GZ, Wu AM, DiStefano JJ (2005) A predictive model of therapeutic monoclonal antibody dynamics and regulation by the neonatal Fc receptor (FcRn). Ann Biomed Eng 33(11):1640–1652
Garg A, Balthasar JP (2007) Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn 34(5):687–709. https://doi.org/10.1007/s10928-007-9065-1
Shah DK, Betts AM (2012) Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn 39(1):67–86. https://doi.org/10.1007/s10928-011-9232-2
Glassman PM, Balthasar JP (2019) Physiologically-based modeling of monoclonal antibody pharmacokinetics in drug discovery and development. Drug Metab Pharmacokinet 34(1):3–13
Lindauer A, Valiathan C, Mehta K, Sriram V, de Greef R, Elassaiss-Schaap J et al (2017) Translational pharmacokinetic/pharmacodynamic modeling of tumor growth inhibition supports dose-range selection of the anti–PD-1 antibody pembrolizumab. CPT: Pharmacomet Syst Pharmacol 6(1):11–20
Khot A, Tibbitts J, Rock D, Shah DK (2017) Development of a translational physiologically based pharmacokinetic model for antibody-drug conjugates: a case study with T-DM1. AAPS J 19(6):1715–1734
Shah DK, Haddish-Berhane N, Betts A (2012) Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin. J Pharmacokinet Pharmacodyn 39(6):643–659
Li Z, Li Y, Chang H-P, Chang H-Y, Guo L, Shah DK (2019) Effect of size on solid tumor disposition of protein therapeutics. Drug Metab Dispos 47(10):1136–1145
Singh AP, Maass KF, Betts AM, Wittrup KD, Kulkarni C, King LE et al (2016) Evolution of antibody-drug conjugate tumor disposition model to predict preclinical tumor pharmacokinetics of trastuzumab-emtansine (T-DM1). AAPS J 18(4):861–875
Van de Watering FC, Rijpkema M, Perk L, Brinkmann U, Oyen WJ, Boerman OC (2014) Zirconium-89 labeled antibodies: a new tool for molecular imaging in cancer patients. BioMed Res Int 2014:1–13
van Dongen G, Beaino W, Windhorst AD, Zwezerijnen GJC, Oprea-Lager DE, Hendrikse NH et al (2021) The role of (89)Zr-immuno-PET in navigating and derisking the development of biopharmaceuticals. J Nucl Med 62(4):438–445. https://doi.org/10.2967/jnumed.119.239558
Eigenmann MJ, Fronton L, Grimm HP, Otteneder MB, Krippendorff B-F (2017) Quantification of IgG monoclonal antibody clearance in tissues. Taylor & Francis, MAbs, pp 1007–1015
Burvenich IJ, Lee F-T, Guo N, Gan HK, Rigopoulos A, Parslow AC et al (2016) In vitro and in vivo evaluation of 89zr-DS-8273a as a theranostic for anti-death receptor 5 therapy. Theranostics 6(12):2225
England CG, Ehlerding EB, Hernandez R, Rekoske BT, Graves SA, Sun H et al (2017) Preclinical pharmacokinetics and biodistribution studies of 89Zr-labeled pembrolizumab. J Nucl Med 58(1):162–168
Cheal SM, Punzalan B, Doran MG, Evans MJ, Osborne JR, Lewis JS et al (2014) Pairwise comparison of 89 Zr-and 124 I-labeled cG250 based on positron emission tomography imaging and nonlinear immunokinetic modeling: in vivo carbonic anhydrase IX receptor binding and internalization in mouse xenografts of clear-cell renal cell carcinoma. Eur J Nucl Med Mol Imaging 41(5):985–994
Holland JP, Caldas-Lopes E, Divilov V, Longo VA, Taldone T, Zatorska D et al (2010) Measuring the pharmacodynamic effects of a novel Hsp90 inhibitor on HER2/neu expression in mice using 89 Zr-DFO-trastuzumab. PLoS ONE 5(1):e8859
Tinianow JN, Gill HS, Ogasawara A, Flores JE, Vanderbilt AN, Luis E et al (2010) Site-specifically 89Zr-labeled monoclonal antibodies for ImmunoPET. Nucl Med Biol 37(3):289–297
Vugts DJ, Heuveling DA, Stigter-van Walsum M, Weigand S, Bergstrom M, van Dongen GA et al (2014) Preclinical evaluation of 89Zr-labeled anti-CD44 monoclonal antibody RG7356 in mice and cynomolgus monkeys: prelude to phase 1 clinical studies. Taylor & Francis, MAbs, pp 567–575
Fissers J, Waldron A-M, De Vijlder T, Van Broeck B, Pemberton DJ, Mercken M et al (2016) Synthesis and evaluation of a Zr-89-labeled monoclonal antibody for immuno-PET imaging of amyloid-β deposition in the brain. Mol Imag Biol 18(4):598–605
Nayak TK, Garmestani K, Milenic DE, Brechbiel MW (2012) PET and MRI of metastatic peritoneal and pulmonary colorectal cancer in mice with human epidermal growth factor receptor 1-targeted 89Zr-labeled panitumumab. J Nucl Med 53(1):113–120. https://doi.org/10.2967/jnumed.111.094169
Chang H-P, Kim SJ, Shah DK (2020) Whole-Body pharmacokinetics of antibody in mice determined using enzyme-linked immunosorbent assay and derivation of tissue interstitial concentrations. J Pharm Sci 110:446
Brouwers A, Verel I, Van Eerd J, Visser G, Steffens M, Oosterwijk E et al (2004) PET radioimmunoscintigraphy of renal cell cancer using 89Zr-labeled cG250 monoclonal antibody in nude rats. Cancer Biother Radiopharm 19(2):155–163
Cole EL, Kim J, Donnelly DJ, Smith RA, Cohen D, Lafont V et al (2017) Radiosynthesis and preclinical PET evaluation of 89Zr-nivolumab (BMS-936558) in healthy non-human primates. Bioorg Med Chem 25(20):5407–5414
Pandit-Taskar N, O’Donoghue JA, Beylergil V, Lyashchenko S, Ruan S, Solomon SB et al (2014) 89 Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur J Nucl Med Mol Imaging 41(11):2093–2105
Jansen MH, van Zanten SEV, Van Vuurden DG, Huisman MC, Vugts DJ, Hoekstra OS et al (2017) Molecular drug imaging: 89Zr-bevacizumab PET in children with diffuse intrinsic pontine glioma. J Nucl Med 58(5):711–716
Jauw YWS, Huisman MC, Nayak TK, Vugts DJ, Christen R, Naegelen VM et al (2018) Assessment of target-mediated uptake with immuno-PET: analysis of a phase I clinical trial with an anti-CD44 antibody. EJNMMI Res 8(1):6. https://doi.org/10.1186/s13550-018-0358-8
Laforest R, Lapi SE, Oyama R, Bose R, Tabchy A, Marquez-Nostra BV et al (2016) [89 Zr] Trastuzumab: evaluation of radiation dosimetry, safety, and optimal imaging parameters in women with HER2-positive breast cancer. Mol Imag Biol 18(6):952–959
Menke-van der Houven CW, van Oordt ECG, Huisman MC, Vugts DJ, Roth C, Luik AM et al (2015) 89Zr-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget 6(30):30384
Börjesson PK, Jauw YW, de Bree R, Roos JC, Castelijns JA, Leemans CR et al (2009) Radiation dosimetry of 89Zr-labeled chimeric monoclonal antibody U36 as used for immuno-PET in head and neck cancer patients. J Nucl Med 50(11):1828–1836
Lindenberg L, Adler S, Turkbey IB, Mertan F, Ton A, Do K et al (2017) Dosimetry and first human experience with 89Zr-panitumumab. Am J Nucl Med Mol Imaging 7(4):195
Li Z, Shah DK (2019) Two-pore physiologically based pharmacokinetic model with de novo derived parameters for predicting plasma PK of different size protein therapeutics. J Pharmacokinet Pharmacodyn 46(3):305–318
Perk LR, Visser GW, Vosjan MJ, Stigter-van Walsum M, Tijink BM, Leemans CR et al (2005) (89)Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals (90)Y and (177)Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J Nucl Med 46(11):1898–1906
Heskamp S, Raave R, Boerman O, Rijpkema M, Goncalves V, Denat F (2017) (89)Zr-immuno-positron emission tomography in oncology: state-of-the-Art (89)Zr radiochemistry. Bioconjug Chem 28(9):2211–2223. https://doi.org/10.1021/acs.bioconjchem.7b00325
Thurber GM, Schmidt MM, Wittrup KD (2008) Factors determining antibody distribution in tumors. Trends Pharmacol Sci 29(2):57–61
Thurber GM, Schmidt MM, Wittrup KD (2008) Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev 60(12):1421–1434
Bensch F, van der Veen EL, Lub-de Hooge MN, Jorritsma-Smit A, Boellaard R, Kok IC et al (2018) 89 Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med 24(12):1852–1858
Harrold JM, Abraham AK (2014) Ubiquity: a framework for physiological/mechanism-based pharmacokinetic/pharmacodynamic model development and deployment. J Pharmacokinet Pharmacodyn 41(2):141–151. https://doi.org/10.1007/s10928-014-9352-6
Buckeridge C, Duvvuri S, Denney WS (2015) Simple, automatic noncompartmental analysis: the PKNCA R package. J Pharmacokinet Pharmacodyn 42(1):11–107
Perk LR, Stigter-van Walsum M, Visser GW, Kloet RW, Vosjan MJ, Leemans CR et al (2008) Quantitative PET imaging of Met-expressing human cancer xenografts with 89 Zr-labelled monoclonal antibody DN30. Eur J Nucl Med Mol Imaging 35(10):1857–1867
Verel I, Visser GW, Boerman OC, Van Eerd JE, Finn R, Boellaard R et al (2003) Long-lived positron emitters zirconium-89 and iodine-124 for scouting of therapeutic radioimmunoconjugates with PET. Cancer Biother Radiopharm 18(4):655–661
Perk LR, Visser GW, Vosjan MJ, Stigter-van Walsum M, Tijink BM, Leemans CR et al (2005) 89Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals 90Y and 177Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J Nucl Med 46(11):1898–1906
Deri MA, Zeglis BM, Francesconi LC, Lewis JS (2013) PET imaging with 89Zr: from radiochemistry to the clinic. Nucl Med Biol 40(1):3–14
Abou DS, Ku T, Smith-Jones PM (2011) In vivo biodistribution and accumulation of 89Zr in mice. Nucl Med Biol 38(5):675–681. https://doi.org/10.1016/j.nucmedbio.2010.12.011
Rafidi H, Estevez A, Ferl GZ, Mandikian D, Stainton S, Sermeno L et al (2021) Imaging reveals importance of shape and flexibility for glomerular filtration of biologics. Mol Cancer Ther 20(10):2008–2015. https://doi.org/10.1158/1535-7163.MCT-21-0116
Liu S, Verma A, Kettenberger H, Richter WF, Shah DK (2021) Effect of variable domain charge on in vitro and in vivo disposition of monoclonal antibodies. MAbs 13(1):1993769. https://doi.org/10.1080/19420862.2021.1993769
Bensch F, Lamberts LE, Smeenk MM, Jorritsma-Smit A, Lub-de Hooge MN, Terwisscha van Scheltinga AGT et al (2017) (89)Zr-Lumretuzumab PET imaging before and during HER3 antibody lumretuzumab treatment in patients with solid tumors. Clin Cancer Res 23(20):6128–6137. https://doi.org/10.1158/1078-0432.CCR-17-0311
Jauw YWS, O’Donoghue JA, Zijlstra JM, Hoekstra OS, der Houven M, van Oordt CW, Morschhauser F et al (2019) (89)Zr-Immuno-PET: toward a noninvasive clinical tool to measure target engagement of therapeutic antibodies in vivo. J Nucl Med 60(12):1825–1832. https://doi.org/10.2967/jnumed.118.224568
der Houven M, van Oordt CW, McGeoch A, Bergstrom M, McSherry I, Smith DA, Cleveland M et al (2019) Immuno-PET imaging to assess target engagement: experience from (89)Zr-anti-HER3 mAb (GSK2849330) in patients with solid tumors. J Nucl Med 60(7):902–909. https://doi.org/10.2967/jnumed.118.214726
Huisman MC, der Houven M, van Oordt CW, Zijlstra JM, Hoekstra OS, Boellaard R, van Dongen G et al (2021) Potential and pitfalls of (89)Zr-immuno-PET to assess target status: (89)Zr-trastuzumab as an example. EJNMMI Res 11(1):74. https://doi.org/10.1186/s13550-021-00813-7
Sepp A, Meno-Tetang G, Weber A, Sanderson A, Schon O, Berges A (2019) Computer-assembled cross-species/cross-modalities two-pore physiologically based pharmacokinetic model for biologics in mice and rats. J Pharmacokinet Pharmacodyn 46(4):339–359. https://doi.org/10.1007/s10928-019-09640-9
Kang Y-k, Han JH, Lim SY, Lee W-W (2017) Glomerular filtration rate measurement in rats using F-18 sodium fluoride dynamic PET/CT: a single compartment model approach. Soc Nucl Med 58:524
Iwama R, Sato T, Sakurai K, Takasuna K, Ichijo T, Furuhama K et al (2014) Estimation of glomerular filtration rate in cynomolgus monkeys (Macaca fascicularis). J Vet Med Sci 76(10):1423–1426. https://doi.org/10.1292/jvms.14-0218
Wang Z, Wang G, Ren J (2022) Using a mathematical modeling to simulate pharmacokinetics and urinary glucose excretion of luseogliflozin and explore the role of SGLT1/2 in renal glucose reabsorption. ACS Omega 7(51):48427–48437. https://doi.org/10.1021/acsomega.2c06483
Schmidt MM, Wittrup KD (2009) A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther 8(10):2861–2871
Young DS, Findlay HP, Hahn SE, Cechetto LM, McConkey F, Vasquez M. Cytotoxicity mediation of cells evidencing surface expression of CD44. Google Patents; 2011.
Maass KF, Kulkarni C, Betts AM, Wittrup KD (2016) Determination of cellular processing rates for a trastuzumab-maytansinoid antibody-drug conjugate (ADC) highlights key parameters for ADC design. AAPS J 18(3):635–646
Zhang S, Wu CC, Fecteau JF, Cui B, Chen L, Zhang L et al (2013) Targeting chronic lymphocytic leukemia cells with a humanized monoclonal antibody specific for CD44. Proc Natl Acad Sci USA 110(15):6127–6132. https://doi.org/10.1073/pnas.1221841110
Acknowledgements
Authors would also like to acknowledge Dr. Yvonne Jauwn from Amsterdam VUMC for her help with interpretation of PET imaging data collected from the clinic.
Funding
This research was funded by the grant from the Center for Protein Therapeutics (CPT) at the University at Buffalo. DKS is also supported by National Institute of General Medical Sciences Grant [GM114179], National Institute of Allergy and Infectious Diseases Grant [AI138195], and National Cancer Institute grants [R01CA246785 and R01CA256928].
Author information
Authors and Affiliations
Contributions
Participated in research design: ZL, SL, MH, DKS. Conducted experiments: ZL, SL. Contributed new reagents or analytic tools: ZL, SL, MH. Performed data analysis: ZL, SL, MH, DKS. Wrote or contributed to the writing of the manuscript: ZL, SL, MH, YJ, DKS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Li, Z., Huisman, M. et al. Clinical validation of translational antibody PBPK model using tissue distribution data generated with 89Zr-immuno-PET imaging. J Pharmacokinet Pharmacodyn 50, 377–394 (2023). https://doi.org/10.1007/s10928-023-09869-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-023-09869-5