Abstract
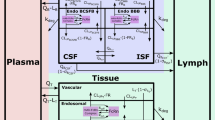
In this manuscript, we have presented the development of a novel platform physiologically-based pharmacokinetic (PBPK) model to characterize brain disposition of mAbs in the mouse, rat, monkey and human. The model accounts for known anatomy and physiology of the brain, including the presence of distinct blood–brain barrier and blood–cerebrospinal fluid (CSF) barrier. CSF and interstitial fluid turnover, and FcRn mediated transport of mAbs are accounted for. The model was first used to characterize published and in-house pharmacokinetic (PK) data on the disposition of mAbs in rat brain, including the data on PK of mAb in different regions of brain determined using microdialysis. Majority of model parameters were fixed based on literature reported values, and only 3 parameters were estimated using rat data. The rat PBPK model was translated to mouse, monkey, and human, simply by changing the values of physiological parameters corresponding to each species. The translated PBPK models were validated by a priori predicting brain PK of mAbs in all three species, and comparing predicted exposures with observed data. The platform PBPK model was able to a priori predict all the validation PK profiles reasonably well (within threefold), without estimating any parameters. As such, the platform PBPK model presented here provides an unprecedented quantitative tool for prediction of mAb PK at the site-of-action in the brain, and preclinical-to-clinical translation of mAbs being developed against central nervous system (CNS) disorders. The proposed model can be further expanded to account for target engagement, disease pathophysiology, and novel mechanisms, to support discovery and development of novel CNS targeting mAbs.






Similar content being viewed by others
References
Pardridge WM (2016) CSF, blood–brain barrier, and brain drug delivery. Expert Opin Drug Deliv 13:963–975
Neves V, Aires-da-Silva F, Corte-Real S, Castanho MARB (2016) Antibody approaches to treat brain diseases. Trends Biotechnol 34:36–48
Dostalek M, Gardner I, Gurbaxani BM, Rose RH, Chetty M (2013) Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet 52:83–124
Matsuo E, Shin R, Billingsley M, Devoorde A, Oconnor M, Trojanowski J, Lee V (1994) Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimers disease paired helical filament tau. Neuron 13:989–1002
Hillered L, Vespa P, Hovda D (2005) Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J Neurotrauma 22:3–41
Pestalozzi BC, Brignoli S (2000) Trastuzumab in CSF. J Clin Oncol 18:2349–2351
Stemmler H, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V (2007) Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood–brain barrier. Anticancer Drugs 18:23–28
Rubenstein J, Fridlyand J, Abrey L, Shen A, Karch J, Wang E (2007) Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 25:1350–1356
Nau R, Sorgel F, Eiffert H (2010) Penetration of drugs through the blood–cerebrospinal fluid/blood–brain barrier for treatment of central nervous system infections. Clin Microbiol Rev 23:858–883
Martín-garcía E, Mannara F, Gutiérrez-cuesta J, Sabater L, Dalmau J, Maldonado R, Graus F (2013) Intrathecal injection of P/Q type voltage-gated calcium channel antibodies from paraneoplastic cerebellar degeneration cause ataxia in mice. J Neuroimmunol 261:53–59
Wolak DJ, Pizzo ME, and Thorne RG (2015) Probing the extracellular diffusion of antibodies in brain using in vivo integrative optical imaging and ex vivo fluorescence imaging. J Control Release. Elsevier B.V.; 197:78–86.
Yadav DB, Maloney JA, Wildsmith KR, Fuji RN, Meilandt WJ, Solanoy H, Lu Y, Peng K, Wilson B, Chan P, Gadkar K, Kosky A, Goo M, Daugherty A, Couch JA, Keene T, Hayes K, Nikolas LJ, Lane D, Switzer R, Adams E, Watts RJ, Levie KS, Prabhu S, Shafer L, Thakker DR, Hildebrand K, Atwal JK (2017) Widespread brain distribution and activity following anti-BACE1 intracerebroventricular infusion in nonhuman primates. Br J Pharmacol 174:4173–4185
Chang H-Y, Morrow K, Bonacquisti E, Zhang WY, Shah DK (2018) Antibody pharmacokinetics in rat brain determined using microdialysis. MAbs 10:843–853
Gadkar K, Yadav DB, Zuchero JY, Couch JA, Kanodia J, Kenrick MK, Atwal JK, Dennis MS, Prabhu S, Watts RJ, Joseph SB, Ramanujan S (2016) Mathematical PKPD and safety model of bispecific TfR/BACE1 antibodies for the optimization of antibody uptake in brain. Eur J Pharm Biopharm 101:53–61
Kanodia J, Gadkar K, Bumbaca D, Zhang Y, Tong R, Luk W, Hoyte K, Lu Y, Wildsmith K, Couch J, Watts R, Dennis M, Ernst J, Scearce-Levie K, Atwal J, Ramanujan S, Joseph S (2016) Prospective design of anti-transferrin receptor bispecific antibodies for optimal delivery into the human brain. CPT Pharmacometrics Syst Pharmacol 5:283–291
Paris-robidas S, Emond V, Tremblay C, Soulet D (2011) In vivo labeling of brain capillary endothelial cells after intravenous injection of monoclonal antibodies targeting the transferrin receptor. Mol Pharmacol 80:32–39
Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, Atwal J, Elliott JM, Prabhu S, Watts RJ, and Dennis MS (2011) Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med 3:84ra44–84ra44.
Moos T, Morgan EH (2001) Restricted transport of anti-transferrin receptor antibody ( OX26) through the blood–brain barrier in the rat. J Neurochem 79:119–129
Pardaidge WM, Buciak JL, Friden PM (1991) Selective transport of an anti-transferrin through the blood–brain barrier in vivo receptor antibody. J Pharmacol Exp Ther 259:66–70
Bien-ly N, Yu YJ, Bumbaca D, Elstrott J, Boswell CA, Zhang Y, Luk W, Lu Y, Dennis MS, Weimer RM, Chung I, Watts RJ (2014) Transferrin receptor (TfR) trafficking determines brain uptake of Tf R antibody affinity variants. J Exp Med 211:233–244
Covell DG, Barbet J, Holton OD, Black CDV, Parker RJ, Weinstein JN (1986) Pharmacokinetics of monoclonal immunoglobulin G1, F(ab’)2 and Fab’ in mice. Cancer Res 46:3969–3978
Mackensen DG, Jain RK (1994) Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res 54:1517–1528
Baxter LT, Zhu H, Jain RK, Zhu H, Mackensen DG, Butler WF (1995) Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res 55:4611–4622
Ferl GZ, Wu AM, DiStefano JJ (2005) A predictive model of therapeutic monoclonal antibody dynamics and regulation by the neonatal Fc receptor (FcRn). Ann Biomed Eng 33:1640–1652
Garg A, Balthasar JP (2007) Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn 34:687–709
Cao Y, Jusko WJ (2012) Applications of minimal physiologically-based pharmacokinetic models. J Pharmacokinet Pharmacodyn 39:711–723
Shah DK, Betts AM (2012) Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn 39:67–86
Cao Y, Balthasar JP, Jusko WJ (2013) Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokinet Pharmacodyn 40:597–607
Glassman PM, Balthasar JP (2017) Physiologically-based modeling to predict the clinical behavior of monoclonal antibodies directed against lymphocyte antigens. MAbs 9:297–306
Brinker T, Stopa E, Morrison J, Klinge P (2014) A new look at cerebrospinal fluid movement. Fluids Barriers CNS 11:1–16
Plog B, Nedergaard M (2017) The glymphatic system in central nervous system health and disease: past, present, and future. Ann Rev Pathol 13:379–394
Hladky SB, Barrand MA (2014) Mechanisms of fluid movement into, through and out of the brain : evaluation of the evidence. Fluids Barriers CNS 11:1–32
Abuqayyas L, Balthasar JP (2013) Investigation of the role of FcγR and FcRn in mAb distribution to the brain. Mol Pharm 10:1505–1513
Garg A, Balthasar JP (2009) Investigation of the influence of FcRn on the distribution of IgG to the brain. AAPS J 11:553–557
Schlachetzki F, Zhu C, Pardridge W (2002) Expression of the neonatal Fc receptor (FcRn) at the blood–brain barrier. J Neurochem 81:203–206
Zhang Y, Pardridge WM (2001) Mediated efflux of IgG molecules from brain to blood across the blood–brain barrier. J Neuroimmunol 114:168–172
Cooper PR, Ciambrone GJ, Kliwinski CM, Maze E, Johnson L, Li Q, Feng Y, Hornby PJ (2013) Efflux of monoclonal antibodies from rat brain by neonatal Fc receptor, FcRn. Brain Res 1534:13–21
Wang Q, Delva L, Weinreb PH, Pepinsky RB, Graham D, Veizaj E, Cheung AE, Chen W, Nestorov I, Rohde E, Caputo R, Kuesters GM, Bohnert T, Gan LS (2018) Monoclonal antibody exposure in rat and cynomolgus monkey cerebrospinal fluid following systemic administration. Fluids Barriers CNS 15:1–10
Noguchi Y, Kato M, Ozeki K, Ishigai M (2017) Pharmacokinetics of an intracerebroventricularly administered antibody in rats. MAbs 9:1210–1215
Bergman I, Burckart GJ, Pohl CR, Venkataramanan R, Barmada MA, Griffin JA, Cheung N-K (1998) Pharmacokinetics of IgG and IgM anti-ganglioside antibodies in rats and monkeys after intrathecal administration. J Pharmacol Exp Ther 284:111–115
Atwal JK, Chen Y, Chiu C, Mortensen DL, Meilandt WJ, Liu Y, Heise CE, Hoyte K, Luk W, Lu Y, Peng K, Wu P, Rouge L, Zhang Y, Lazarus RA, Scearce-Levie K, Wang W, Wu Y, Tessier-Lavigne M, Watts RJ.(2011) A therapeutic antibody targeting BACE1 inhibits amyloid-β production in vivo. Sci Transl Med 3(84):84ra43.
Braen APJM, Perron J, Tellier P, Catala AR, Kolaitis G, Geng W (2010) A 4-week intrathecal toxicity and pharmacokinetic study with trastuzumab in cynomolgus monkeys. Int J Toxicol 29:259–267
Kaschka WP, Theilkaes L, Eickhoff K, Skvaril F (1979) Disproportionate elevation of the immunoglobulin G1 concentration in cerebrospinal fluids of patients with multiple sclerosis. Infect Immun 26:933–941
Curtin F, Vidal V, Bernard C, Kromminga A, Lang AB, Porchet H (2016) Serum pharmacokinetics and cerebrospinal fluid concentration analysis of the new IgG4 monoclonal antibody GNbAC1 to treat multiple sclerosis: a phase 1 study. MAbs 8:854–860
Borvak J, Richardson J, Medesan C, Antohe F, Radu C, Simionescu M, Ghetie V, Ward E (1998) Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol 101289–1298(10):1289–1298
Cauza K, Hinterhuber G, Dingelmaier-Hovorka R, Brugger K, Klosner G, Horvat R, Wolff K, Foedinger D (2005) Expression of FcRn, the MHC class I-related receptor for IgG, in human keratinocytes. J Invest Dermatol 124:132–139
Blumberg R, Koss T, Story C, Barisani D, Polischuk J, Lipin A, Pablo L, Green R, Simister N (1995) A major histocompatibility complex class I-related Fc receptor for IgG on rat hepatocytes. J Clin Invest 95:2397–2402
Cianga P, Cianga C, Cozma L, Ward E, Carasevici E (2003) The MHC class I related Fc receptor, FcRn, is expressed in the epithelial cells of the human mammary gland. Hum Immunol 64:1152–1159
Akilesh S, Christianson G, Roopenian D, Shaw A (2007) Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol 179:4580–4588
Garg A (2007) Investigation of the role of FcRn in the absorption, distribution, and elimination of monoclonal antibodies, Chap 3. PhD Thesis, Dep Pharm Sci., pp. 71–111.
Hladky SB, Barrand MA (2016) Fluid and ion transfer across the blood–brain and blood–cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 13:1–69
Villasenõr R, Ozmen L, Messaddeq N, Grüninger F, Loetscher H, Keller A, Betsholtz C, Freskgård PO, Collin L (2016) Trafficking of endogenous immunoglobulins by endothelial cells at the blood–brain barrier. Sci Rep 6:1–10
Urva SR, Yang VC, Balthasar JP (2010) Physiologically based pharmacokinetic model for T84.66: a monoclonal anti-CEA antibody. J Pharm Sci 99:1582–1600
Davson H, and Segal MB (1996) Physiology of the CSF and blood–brain barriers. CRC Press, Boca Raton
Pizzo ME, Wolak DJ, Kumar NN, Brunette E, Brunnquell CL, Hannocks MJ, Abbott NJ, Meyerand ME, Sorokin L, Stanimirovic DB, Thorne RG (2018) Intrathecal antibody distribution in the rat brain: surface diffusion, perivascular transport and osmotic enhancement of delivery. J Physiol 596:445–475
Sarin H (2012) Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes 2:1–19
Khot A, Tibbitts J, Rock D, Shah DK (2017) Development of a translational physiologically based pharmacokinetic model for antibody-drug conjugates: a case study with T-DM1. AAPS J 19:1715–1734
Sobol IM (2001) Global sensitivity indices for nonlinear mathematical models and their Monte Carlo estimates. Math Comput Simulat 55:271–280
Wolburg H, Lippoldt A (2002) Tight junctions of the blood–brain barrier : development, composition and regulation. Vascul Pharmacol 38:323–337
Roopenian DC, Akilesh S (2007) FcRn : the neonatal Fc receptor comes of age. Nat Rev Immunol 7:715–725
St-Amour I, Pare I, Alata W, Coulombe K, Ringuette-goulet C, Drouin-ouellet J, Vandal M, Soulet D, Bazin R, Calon F (2013) Brain bioavailability of human intravenous immunoglobulin and its transport through the murine blood–brain barrier. J Cereb Blood Flow Metab 33:1983–1992
Simon MJ, Iliff JJ (2016) Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta 1862:442–451
Cserr H (1971) Physiology of choroid plexus. Physiol Rev 51:273–311
Damkier H, Brown P, Praetorius J (2013) Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev 93:1847–1892
Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, Da Mesquita S, Frost EL, Gaultier A, Harris TH, Cao R, Hu S, Lukens JR, Smirnov I, Overall CC, Oliver G, Kipnis J (2018) CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 21:1380–1391
Mesquita S Da, Fu Z, and Kipnis J (2018) The meningeal lymphatic system: a new player in neurophysiology. Neuron 100:375–88.
Levy G (1994) Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther 56:248–252
Deane R, Sagare A, Hamm K, Parisi M, LaRue B, Guo H, Wu Z, Holtzman DM, Zlokovic BV (2005) IgG-assisted age-dependent clearance of Alzheimer’s amyloid peptide by the blood–brain barrier neonatal Fc receptor. J Neurosci 25:11495–11503
Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T (2000) Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6:916–919
Collin L, Bohrmann B, Gopfert U, Oroszlan-szovik K, Ozmen L, Gruninger F (2014) Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer’s disease. Brain 137:2834–2846
Thom George, Burrell M, Haqqani AS, Yogi A, Lessard E, Brunette E, Delaney C, Baumann E, Callaghan D, Rodrigo N, Webster CI, Stanimirovic DB (2018) Enhanced delivery of galanin conjugates to the brain through bioengineering of the anti-transferrin receptor antibody OX26. Mol Pharm 15:1420–1431
Yu YJ, Atwal JK, Zhang Y, Tong RK, Wildsmith KR, Tan C, Bien-Ly N, Hersom M, Maloney JA, Meilandt WJ, Bumbaca D, Gadkar K, Hoyte K, Luk W, Lu Y, Ernst JA, Scearce-Levie K, Couch JA, Dennis MS, Watts RJ. (2014) Therapeutic bispecific antibodies cross the blood–brain barrier in nonhuman primates. Sci Transl Med 6:261ra154.
Couch JA, Yu YJ, Zhang Y, Tarrant JM, Fuji RN, Meilandt WJ, Solanoy H, Tong RK, Hoyte K, Luk W, Lu Y, Gadkar K, Prabhu S, Ordonia BA, Nguyen Q, Lin Y, Lin Z, Balazs M, Scearce-Levie K, Ernst JA, Dennis MS, Watts RJ. (2013) Addressing safety liabilities of TfR bispecific antibodies that cross the blood–brain barrier. Sci Transl Med 5:183ra57,1–12.
Okuyama T, Sakai N, Yamamoto T, Yamaoka M, Tomio T (2018) Novel blood–brain barrier delivery system to treat CNS in MPS II: first clinical trial of anti-transferrin receptor antibody fused enzyme therapy. Mol Genet Metab 123:S109
Oshio K, Watanabe H, Song Y, Verkman A, Manley G (2005) Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J 19:76–78
de Lange ECM (2013) Utility of CSF in translational neuroscience. J Pharmacokinet Pharmacodyn 40:315–326
Kumar G, Smith Q, Hokari M, Parepally J, Duncan M (2007) Brain uptake, pharmacokinetics, and tissue distribution in the rat of neurotoxic N-butylbenzenesulfonamide. Toxicol Sci 98:607–609
Rudick R, Zirretta D, Herndon R (1982) Clearance of albumin from mouse subarachnoid space: a measure of CSF bulk flow. J Neurosci Methods 6:253–259
Yamamoto Y, Välitalo PA, Wong YC, Huntjens DR, Proost JH, Vermeulen A, Krauwinkel W, Beukers MW, Kokki H, Kokki M, Danhof M, van Hasselt JGC, de Lange ECM (2018) Prediction of human CNS pharmacokinetics using a physiologically-based pharmacokinetic modeling approach. Eur J Pharm Sci 112:168–179
Ridgway J, Turnbull L, Smith M (1987) Demonstration of pulsatile cerebrospinal-fluid flow using magnetic resonance phase imaging. Br J Radiol 60:423–427
Westerhout J, Ploeger B, Smeets J, Danhof M, de Lange ECM (2012) Physiologically based pharmacokinetic modeling to investigate regional brain distribution kinetics in rats. AAPS J 14:543–553
Abbott N (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45:545–552
Szentistványi I, Patlak C, Ellis R, Cserr H (1984) Drainage of interstitial fluid from different regions of rat brain. Am J Physiol 246:F835–844
Kovačević N, Henderson J, Chan E, Lifshitz N, Bishop J, Evans A, Henkelman R, Chen X (2005) A Three-dimensional MRI Atlas of the Mouse Brain with Estimates of the Average and Variability. Cereb Cortex 15:639–645
Yamamoto Y, Välitalo PA, Huntjens DR, Proost JH, Vermeulen A, Krauwinkel W, Beukers MW, Van Den Berg DJ, Hartman R, Wong YC, Danhof M, Van Hasselt JGC, de Lange ECM (2017) Predicting drug concentration-time profiles in multiple CNS compartments using a comprehensive physiologically-based pharmacokinetic model. CPT Pharmacometrics Syst Pharmacol 6:765–777
Dorr AE, Lerch JP, Spring S, Kabani N, Henkelman RM (2008) High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 42:60–69
Strazielle N, Ghersi-Egea JF (2000) Choroid plexus in the central nervous system: biology and physiopathology. J Neuropathol Exp Neurol 59:561–574
Johanson CE, Duncan JA, Klinge PM, Brinker T, Stopa EG, Silverberg GD (2008) Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res 5:1–32
Silverberg G, Heit G, Huhn S, Jaffe R, Chang S, Bronte-Stewart H, Rubenstein E, Possin K, Saul T (2001) The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology 57:1763–1766
Acknowledgements
This work was supported by the Centre for Protein Therapeutics at University at Buffalo. D.K.S is supported by National Institute of Health Grant [GM114179] and [AI138195].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
A glossary of parameters used to build the platform brain PBPK model for mAbs
Parameter | Units | Definition |
|---|---|---|
\({Q}_{p}^{i}\) | L/h | Plasma flow to the tissue “i” |
\({Q}_{BC}^{i}\) | L/h | Blood cell flow to the tissue “i” |
\({L}^{i}\) | L/h | Lymph flow from the tissue “i” |
\({Q}_{CSF}^{brain},\) \({Q}_{ISF}^{brain}\) | L/h | CSF and brain interstitial fluid formation rate |
\({V}^{p}\), \({V}^{BC}\), \({V}^{LN}\) | L | Volume of central plasma, central blood cell and lymph node compartments |
\({V}_{BBB}^{brain}\), \({V}_{BCSFB}^{brain}\), \({V}_{LV}^{brain}\), \({V}_{TFV}^{brain}\), \({V}_{CM}^{brain}\), \({V}_{SAS(LS)}^{brain}\) | L | Volume of BBB endosomal, BCSFB endosomal, LV, TFV, CM and SAS compartments |
\({V}_{p}^{i}\), \({V}_{BC}^{i}, {V}_{ES}^{i}\), \({V}_{IS}^{i}\) | L | Volume of vascular, blood cell, endosomal, and interstitial compartments for tissue “i” |
\({C}^{p}\), \({C}^{BC}\), \({C}^{LN}\) | M | Concentration of mAb in central plasma, central blood cell and lymph node compartments |
\({C}_{{BBB}_{unbound}}^{brain}\), \({C}_{{BBB}_{bound}}^{brain}\), \({C}_{{BCSFB}_{unbound}}^{brain}\), \({{C}_{{BCSFB}_{bound}}^{brain}}\), \({C}_{LV}^{brain}\), \({{C}_{TFV}^{brain}}\), \({C}_{CM}^{brain}\), \({C}_{SAS(LS)}^{brain}\) | M | Concentration of mAb in BBB endosomal (Unbound and Bound), BCSFB endosomal (Unbound and Bound), LV, TFV, CM and SAS compartments |
\({C}_{p}^{i}\), \({C}_{BC}^{i}\), \({C}_{E\_unbound}^{i}\), \({C}_{E\_bound}^{i}\), \({C}_{IS}^{i}\) | M | Concentration of mAb in vascular, blood cell, endosomal (Unbound and Bound), interstitial and cellular (Bound) compartments for tissue “i” |
\({FcRn}_{free}^{i}\) | M | Concentration of free FcRn in endosomal space |
\({\sigma }_{V}^{i}\), \({\sigma }_{L}^{i}\) | – | Vascular and lymph reflection coefficient |
\({\sigma }_{V}^{BBB}\), \({{\sigma }_{V}^{BCSFB}}\) | – | BBB and BCSFB vascular reflection coefficient |
\({k}_{on}\) | 1/M/h | Association rate constant between mAb-FcRn |
\({k}_{off}\) | 1/h | Dissociation rate constant between mAb-FcRn |
FR | – | Fraction of FcRn bound mAb that recycles to the vascular space |
\({f}_{BBB},\) \({f}_{BCSFB}\) | – | Surface area fractions of BBB and BCSFB |
\({f}_{LV}\), \({f}_{TFV}\) | – | Volume fractions of LV and TFV |
\(S{A}_{BBB}\), \(S{A}_{BCSFB}\) | L | Surface area of BBB and BCSFB |
\(C{L}_{up}\), \({C{L}_{up}^{brain}}\) | L/h/L | Rate of pinocytosis and exocytosis per unit endosomal space for tissue and brain |
\({k}_{deg}\) | 1/h | First order degradation rate constant of FcRn unbound mAb within the endosomal space |
Rights and permissions
About this article
Cite this article
Chang, HY., Wu, S., Meno-Tetang, G. et al. A translational platform PBPK model for antibody disposition in the brain. J Pharmacokinet Pharmacodyn 46, 319–338 (2019). https://doi.org/10.1007/s10928-019-09641-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-019-09641-8