Abstract
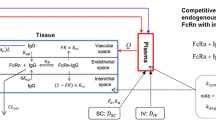
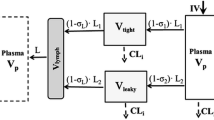
Development of monoclonal antibodies (mAbs) and their functional derivatives represents a growing segment of the development pipeline in the pharmaceutical industry. More than 25 mAbs and derivatives have been approved for a variety of therapeutic applications. In addition, around 500 mAbs and derivatives are currently in different stages of development. mAbs are considered to be large molecule therapeutics (in general, they are 2–3 orders of magnitude larger than small chemical molecule therapeutics), but they are not just big chemicals. These compounds demonstrate much more complex pharmacokinetic and pharmacodynamic behaviour than small molecules. Because of their large size and relatively poor membrane permeability and instability in the conditions of the gastrointestinal tract, parenteral administration is the most usual route of administration. The rate and extent of mAb distribution is very slow and depends on extravasation in tissue, distribution within the particular tissue, and degradation. Elimination primarily happens via catabolism to peptides and amino acids. Although not definitive, work has been published to define the human tissues mainly involved in the elimination of mAbs, and it seems that many cells throughout the body are involved. mAbs can be targeted against many soluble or membrane-bound targets, thus these compounds may act by a variety of mechanisms to achieve their pharmacological effect. mAbs targeting soluble antigen generally exhibit linear elimination, whereas those targeting membrane-bound antigen often exhibit non-linear elimination, mainly due to target-mediated drug disposition (TMDD). The high-affinity interaction of mAbs and their derivatives with the pharmacological target can often result in non-linear pharmacokinetics. Because of species differences (particularly due to differences in target affinity and abundance) in the pharmacokinetics and pharmacodynamics of mAbs, pharmacokinetic/pharmacodynamic modelling of mAbs has been used routinely to expedite the development of mAbs and their derivatives and has been utilized to help in the selection of appropriate dose regimens. Although modelling approaches have helped to explain variability in both pharmacokinetic and pharmacodynamic properties of these drugs, there is a clear need for more complex models to improve understanding of pharmacokinetic processes and pharmacodynamic interactions of mAbs with the immune system. There are different approaches applied to physiologically based pharmacokinetic (PBPK) modelling of mAbs and important differences between the models developed. Some key additional features that need to be accounted for in PBPK models of mAbs are neonatal Fc receptor (FcRn; an important salvage mechanism for antibodies) binding, TMDD and lymph flow. Several models have been described incorporating some or all of these features and the use of PBPK models are expected to expand over the next few years.




Similar content being viewed by others
References
FACTBOX–World’s top-selling drugs in 2014 vs 2010. 2010. Reuters News. 2010 Apr 13. http://www.reuters.com/article/2010/04/13/roche-avastin-drugs-idUSLDE63C0BC20100413. Accessed 6 Nov 2012.
Porter RR. Structural studies of immunoglobulins. Science. 1973;180(4087):713–6.
Edelman GM. Antibody structure and molecular immunology. Science. 1973;180(4088):830–40.
Martin NH. The immunoglobulins: a review. J Clin Pathol. 1969;22(2):117–31.
Waldmann TA, Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110.
Spiegelberg HL, Weigle WO. The catabolism of homologous and heterologous 7 s gamma globulin fragments. J Exp Med. 1965;121:323–38.
Spiegelberg HL, Weigle WO. Studies on the catabolism of γG subunits and chains. J Immunol. 1965;95(6):1034–40.
Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG subclasses in man. J Clin Invest. 1970;49(4):673–80.
Ternant D, Paintaud G. Pharmacokinetics and concentration-effect relationships of therapeutic monoclonal antibodies and fusion proteins. Expert Opin Biol Ther. 2005;5(Suppl 1):S37–47.
Thornton CA, Welle S, Griggs RC, Abraham GN. Human IgG production in vivo: determination of synthetic rate by nonradioactive tracer incorporation. J Immunol. 1996;157(2):950–5.
Imbach P, Barandun S, d’Apuzzo V, Baumgartner C, Hirt A, Morell A, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981;1(8232):1228–31.
Bleeker WK, Teeling JL, Hack CE. Accelerated autoantibody clearance by intravenous immunoglobulin therapy: studies in experimental models to determine the magnitude and time course of the effect. Blood. 2001;98(10):3136–42.
Hansen RJ, Balthasar JP. Intravenous immunoglobulin mediates an increase in anti-platelet antibody clearance via the FcRn receptor. Thromb Haemost. 2002;88(6):898–9.
Gratwohl A, Doran JE, Bachmann P, Scherz R, Spath P, Baumgartner C, et al. Serum concentrations of immunoglobulins and of antibody isotypes in bone marrow transplant recipients treated with high doses of polyspecific immunoglobulin or with cytomegalovirus hyperimmune globulin. Bone Marrow Transplant. 1991;8(4):275–82.
Mellstedt H. Monoclonal antibodies as enhancers of the host’s immunoresponse against the tumour. Ann Oncol. 2000;11(Suppl 3):191–4.
Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6(5):343–57.
Gaspar J, Gerritsen B, Jones A. Immunoglobulin replacement treatment by rapid subcutaneous infusion. Arch Dis Child. 1998;79(1):48–51.
Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–7.
Juweid M, Swayne LC, Sharkey RM, Dunn R, Rubin AD, Herskovic T, et al. Prospects of radioimmunotherapy in epithelial ovarian cancer: results with iodine-131-labeled murine and humanized MN-14 anti-carcinoembryonic antigen monoclonal antibodies. Gynecol Oncol. 1997;67(3):259–71.
Bell SJ, Kamm MA. Review article: the clinical role of anti-TNFalpha antibody treatment in Crohn’s disease. Aliment Pharmacol Ther. 2000;14(5):501–14.
Rehlaender BN, Cho MJ. Antibodies as carrier proteins. Pharm Res. 1998;15(11):1652–6.
Richter WF, Gallati H, Schiller CD. Animal pharmacokinetics of the tumor necrosis factor receptor-immunoglobulin fusion protein lenercept and their extrapolation to humans. Drug Metab Dispos. 1999;27(1):21–5.
Skogh T, Stendahl O, Sundqvist T, Edebo L. Physicochemical properties and blood clearance of human serum albumin conjugated to different extents with dinitrophenyl groups. Int Arch Allergy Appl Immunol. 1983;70(3):238–44.
Breedveld FC. Therapeutic monoclonal antibodies. Lancet. 2000;355(9205):735–40.
Economides AN, Carpenter LR, Rudge JS, Wong V, Koehler-Stec EM, Hartnett C, et al. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat Med. 2003;9(1):47–52.
Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633–59.
Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493–507.
Tabrizi M, Bornstein GG, Suria H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 2010;12(1):33–43.
Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11(1–2):81–8.
Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–58.
Mould DR, Sweeney KR. The pharmacokinetics and pharmacodynamics of monoclonal antibodies–mechanistic modeling applied to drug development. Curr Opin Drug Discov Devel. 2007;10(1):84–96.
Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs. 2010;24(1):23–39.
Mortensen DL, Walicke PA, Wang X, Kwon P, Kuebler P, Gottlieb AB, et al. Pharmacokinetics and pharmacodynamics of multiple weekly subcutaneous efalizumab doses in patients with plaque psoriasis. J Clin Pharmacol. 2005;45(3):286–98.
Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62(5):779–86.
Loegering DJ, Blumenstock FA, Cuddy BG. Determination of Kupffer cell Fc receptor function in vivo following injury. Proc Soc Exp Biol Med. 1989;192(3):255–60.
D’Amato G, Salzillo A, Piccolo A, D’Amato M, Liccardi G. A review of anti-IgE monoclonal antibody (omalizumab) as add on therapy for severe allergic (IgE-mediated) asthma. Ther Clin Risk Manag. 2007;3(4):613–9.
Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14(3):559–70.
Porter CJ, Charman SA. Lymphatic transport of proteins after subcutaneous administration. J Pharm Sci. 2000;89(3):297–310.
Charman SA, Segrave AM, Edwards GA, Porter CJ. Systemic availability and lymphatic transport of human growth hormone administered by subcutaneous injection. J Pharm Sci. 2000;89(2):168–77.
Supersaxo A, Hein WR, Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res. 1990;7(2):167–9.
McLennan DN, Porter CJ, Edwards GA, Martin SW, Heatherington AC, Charman SA. Lymphatic absorption is the primary contributor to the systemic availability of epoetin Alfa following subcutaneous administration to sheep. J Pharmacol Exp Ther. 2005;313(1):345–51.
Porter CJ, Edwards GA, Charman SA. Lymphatic transport of proteins after s.c. injection: implications of animal model selection. Adv Drug Deliv Rev. 2001;50(1–2):157–71.
Guyton AC, Hall JE. Textbook of medical physiology (Guyton physiology). 12th ed. Philadelphia: Elsevier Sauders; 2010.
Mosekilde E, Jensen KS, Binder C, Pramming S, Thorsteinsson B. Modeling absorption kinetics of subcutaneous injected soluble insulin. J Pharmacokinet Biopharm. 1989;17(1):67–87.
Brange J, Volund A. Insulin analogs with improved pharmacokinetic profiles. Adv Drug Deliv Rev. 1999;35(2–3):307–35.
Bocci V, Muscettola M, Naldini A, Bianchi E, Segre G. The lymphatic route–II. Pharmacokinetics of human recombinant interferon-alpha 2 injected with albumin as a retarder in rabbits. Gen Pharmacol. 1986;17(1):93–6.
Kagan L, Turner MR, Balu-Iyer SV, Mager DE. Subcutaneous absorption of monoclonal antibodies: role of dose, site of injection, and injection volume on rituximab pharmacokinetics in rats. Pharm Res. 2012;29(2):490–9.
Ibrahim R, Nitsche JM, Kasting GB. Dermal clearance model for epidermal bioavailability calculations. J Pharm Sci. 2012;101(6):2094–108.
Beshyah SA, Anyaoku V, Niththyananthan R, Sharp P, Johnston DG. The effect of subcutaneous injection site on absorption of human growth hormone: abdomen versus thigh. Clin Endocrinol (Oxf). 1991;35(5):409–12.
Macdougall IC, Jones JM, Robinson MI, Miles JB, Coles GA, Williams JD. Subcutaneous erythropoietin therapy: comparison of three different sites of injection. Contrib Nephrol. 1991;88:152–6. (discussion 7–8).
ter Braak EW, Woodworth JR, Bianchi R, Cerimele B, Erkelens DW, Thijssen JH, et al. Injection site effects on the pharmacokinetics and glucodynamics of insulin lispro and regular insulin. Diabetes Care. 1996;19(12):1437–40.
Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93(11):2645–68.
Vaishnaw AK, TenHoor CN. Pharmacokinetics, biologic activity, and tolerability of alefacept by intravenous and intramuscular administration. J Pharmacokinet Pharmacodyn. 2002;29(5–6):415–26.
McLennan DN, Porter CJ, Edwards GA, Heatherington AC, Martin SW, Charman SA. The absorption of darbepoetin alfa occurs predominantly via the lymphatics following subcutaneous administration to sheep. Pharm Res. 2006;23(9):2060–6.
Losonsky GA, Johnson JP, Winkelstein JA, Yolken RH. Oral administration of human serum immunoglobulin in immunodeficient patients with viral gastroenteritis: a pharmacokinetic and functional analysis. J Clin Invest. 1985;76(6):2362–7.
Guarino A, Canani RB, Russo S, Albano F, Canani MB, Ruggeri FM, et al. Oral immunoglobulins for treatment of acute rotaviral gastroenteritis. Pediatrics. 1994;93(1):12–6.
Flessner MF, Lofthouse J, el Zakaria R. In vivo diffusion of immunoglobulin G in muscle: effects of binding, solute exclusion, and lymphatic removal. Am J Physiol. 1997;273(6 Pt 2):H2783–93.
Baxter LT, Zhu H, Mackensen DG, Jain RK. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994;54(6):1517–28.
Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK. Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res. 1995;55(20):4611–22.
Ferl GZ, Wu AM, DiStefano JJ 3rd. A predictive model of therapeutic monoclonal antibody dynamics and regulation by the neonatal Fc receptor (FcRn). Ann Biomed Eng. 2005;33(11):1640–52.
Jain RK. Delivery of molecular medicine to solid tumors: lessons from in vivo imaging of gene expression and function. J Control Release. 2001;74(1–3):7–25.
Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure: an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–13.
Yokota T, Milenic DE, Whitlow M, Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992;52(12):3402–8.
Pardridge WM. Neurotrophins, neuroprotection and the blood-brain barrier. Curr Opin Investig Drugs. 2002;3(12):1753–7.
Ganrot K, Laurell CB. Measurement of IgG and albumin content of cerebrospinal fluid, and its interpretation. Clin Chem. 1974;20(5):571–3.
Schlachetzki F, Zhu C, Pardridge WM. Expression of the neonatal Fc receptor (FcRn) at the blood-brain barrier. J Neurochem. 2002;81(1):203–6.
Garg A, Balthasar JP. Investigation of the influence of FcRn on the distribution of IgG to the brain. AAPS J. 2009;11(3):553–7.
Deane R, Sagare A, Hamm K, Parisi M, LaRue B, Guo H, et al. IgG-assisted age-dependent clearance of Alzheimer’s amyloid beta peptide by the blood-brain barrier neonatal Fc receptor. J Neurosci. 2005;25(50):11495–503.
Abuqayyas L, Balthasar JP. Investigation of the Role of FcgammaR and FcRn in mAb distribution to the brain. Mol Pharm. 2012 Aug 23.
Triguero D, Buciak JB, Yang J, Pardridge WM. Blood-brain barrier transport of cationized immunoglobulin G: enhanced delivery compared to native protein. Proc Natl Acad Sci USA. 1989;86(12):4761–5.
Triguero D, Buciak JL, Pardridge WM. Cationization of immunoglobulin G results in enhanced organ uptake of the protein after intravenous administration in rats and primate. J Pharmacol Exp Ther. 1991;258(1):186–92.
Kemper EM, Boogerd W, Thuis I, Beijnen JH, van Tellingen O. Modulation of the blood-brain barrier in oncology: therapeutic opportunities for the treatment of brain tumours? Cancer Treat Rev. 2004;30(5):415–23.
Unterberg A, Wahl M, Baethmann A. Effects of bradykinin on permeability and diameter of pial vessels in vivo. J Cereb Blood Flow Metab. 1984;4(4):574–85.
Takakura Y, Fujita T, Hashida M, Sezaki H. Disposition characteristics of macromolecules in tumor-bearing mice. Pharm Res. 1990;7(4):339–46.
Maack T, Johnson V, Kau ST, Figueiredo J, Sigulem D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int. 1979;16(3):251–70.
Ghetie V, Ward ES. FcRn: the MHC class I-related receptor that is more than an IgG transporter. Immunol Today. 1997;18(12):592–8.
Gillies SD, Lo KM, Burger C, Lan Y, Dahl T, Wong WK. Improved circulating half-life and efficacy of an antibody-interleukin 2 immunocytokine based on reduced intracellular proteolysis. Clin Cancer Res. 2002;8(1):210–6.
Meier W, Gill A, Rogge M, Dabora R, Majeau GR, Oleson FB, et al. Immunomodulation by LFA3TIP, an LFA-3/IgG1 fusion protein: cell line dependent glycosylation effects on pharmacokinetics and pharmacodynamic markers. Ther Immunol. 1995;2(3):159–71.
Garg A, Balthasar JP. Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn. 2007;34(5):687–709.
Chen Y, Balthasar JP. Evaluation of a catenary PBPK model for predicting the in vivo disposition of mAbs engineered for high-affinity binding to FcRn. AAPS J. 2012;14(4):850–9.
Wiseman GA, Kornmehl E, Leigh B, Erwin WD, Podoloff DA, Spies S, et al. Radiation dosimetry results and safety correlations from 90Y-ibritumomab tiuxetan radioimmunotherapy for relapsed or refractory non-Hodgkin’s lymphoma: combined data from 4 clinical trials. J Nucl Med. 2003;44(3):465–74.
Hooks MA, Wade CS, Millikan WJ Jr. Muromonab CD-3: a review of its pharmacology, pharmacokinetics, and clinical use in transplantation. Pharmacotherapy. 1991;11(1):26–37.
Thomas SM, Grandis JR. Pharmacokinetic and pharmacodynamic properties of EGFR inhibitors under clinical investigation. Cancer Treat Rev. 2004;30(3):255–68.
Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41(9):1552–63.
Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84(8):2457–66.
Rebello SS, Kasiewski CJ, Bentley RG, Morgan SR, Chu V, Bostwick JS, et al. Superiority of enoxaparin over heparin in combination with a GPIIb/IIIa receptor antagonist during coronary thrombolysis in dogs. Thromb Res. 2001;102(3):261–71.
Vincenti F, Nashan B, Light S. Daclizumab: outcome of phase III trials and mechanism of action: double therapy and the triple therapy study groups. Transplant Proc. 1998;30(5):2155–8.
Bang LM, Plosker GL. Omalizumab: a review of its use in the management of allergic asthma. Treat Respir Med. 2004;3(3):183–99.
Weisman MH, Moreland LW, Furst DE, Weinblatt ME, Keystone EC, Paulus HE, et al. Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther. 2003;25(6):1700–21.
Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24(1):19–28.
Li X, Ptacek TS, Brown EE, Edberg JC. Fcgamma receptors: structure, function and role as genetic risk factors in SLE. Genes Immun. 2009;10(5):380–9.
Capel PJ, van de Winkel JG, van den Herik-Oudijk IE, Verbeek JS. Heterogeneity of human IgG Fc receptors. Immunomethods. 1994;4(1):25–34.
Bournazos S, Woof JM, Hart SP, Dransfield I. Functional and clinical consequences of Fc receptor polymorphic and copy number variants. Clin Exp Immunol. 2009;157(2):244–54.
Cooke GS, Aucan C, Walley AJ, Segal S, Greenwood BM, Kwiatkowski DP, et al. Association of Fcgamma receptor IIa (CD32) polymorphism with severe malaria in West Africa. Am J Trop Med Hyg. 2003;69(6):565–8.
Platonov AE, Kuijper EJ, Vershinina IV, Shipulin GA, Westerdaal N, Fijen CA, et al. Meningococcal disease and polymorphism of FcgammaRIIa (CD32) in late complement component-deficient individuals. Clin Exp Immunol. 1998;111(1):97–101.
Rascu A, Repp R, Westerdaal NA, Kalden JR, van de Winkel JG. Clinical relevance of Fc gamma receptor polymorphisms. Ann NY Acad Sci. 1997;815:282–95.
Binstadt BA, Geha RS, Bonilla FA. IgG Fc receptor polymorphisms in human disease: implications for intravenous immunoglobulin therapy. J Allergy Clin Immunol. 2003;111(4):697–703.
Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–33.
Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337(6203):184–7.
Simister NE, Rees AR. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol. 1985;15(7):733–8.
West AP Jr, Bjorkman PJ. Crystal structure and immunoglobulin G binding properties of the human major histocompatibility complex-related Fc receptor. Biochemistry. 2000;39(32):9698–708.
Burmeister WP, Gastinel LN, Simister NE, Blum ML, Bjorkman PJ. Crystal structure at 2.2 Ǻ resolution of the MHC-related neonatal Fc receptor. Nature. 1994;372(6504):336–43.
Kuo TT, Baker K, Yoshida M, Qiao SW, Aveson VG, Lencer WI, et al. Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol. 2010;30(6):777–89.
Tiwari B, Junghans RP. Functional analysis of the mouse Fcgrt 5′ proximal promoter. Biochim Biophys Acta. 2005;1681(2–3):88–98.
Liu X, Ye L, Christianson GJ, Yang JQ, Roopenian DC, Zhu X. NF-kappaB signaling regulates functional expression of the MHC class I-related neonatal Fc receptor for IgG via intronic binding sequences. J Immunol. 2007;179(5):2999–3011.
Liu X, Ye L, Bai Y, Mojidi H, Simister NE, Zhu X. Activation of the JAK/STAT-1 signaling pathway by IFN-gamma can down-regulate functional expression of the MHC class I-related neonatal Fc receptor for IgG. J Immunol. 2008;181(1):449–63.
Gill RK, Mahmood S, Sodhi CP, Nagpaul JP, Mahmood A. IgG binding and expression of its receptor in rat intestine during postnatal development. Indian J Biochem Biophys. 1999;36(4):252–7.
Capano G, Bloch KJ, Schiffrin EJ, Dascoli JA, Israel EJ, Harmatz PR. Influence of the polyamine, spermidine, on intestinal maturation and dietary antigen uptake in the neonatal rat. J Pediatr Gastroenterol Nutr. 1994;19(1):34–42.
Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I- related receptor FcRn. Annu Rev Immunol. 2000;18:739–66.
Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996;26(3):690–6.
Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. Increased clearance of IgG in mice that lack beta 2-microglobulin: possible protective role of FcRn. Immunology. 1996;89(4):573–8.
Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA. 1996;93(11):5512–6.
Waldmann TA, Terry WD. Familial hypercatabolic hypoproteinemia. A disorder of endogenous catabolism of albumin and immunoglobulin. J Clin Invest. 1990;86(6):2093–8.
Wani MA, Haynes LD, Kim J, Bronson CL, Chaudhury C, Mohanty S, et al. Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant beta2-microglobulin gene. Proc Natl Acad Sci USA. 2006;103(13):5084–9. Erratum in: Proc Natl Acad Sci USA. 2006 Jul 5;103(27):10526.
Rodewald R. pH-Dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J Cell Biol. 1976;71(2):666–9.
Wallace KH, Rees AR. Studies on the immunoglobulin-G Fc-fragment receptor from neonatal rat small intestine. Biochem J. 1980;188(1):9–16.
Gan S, Yang P, Yang W. Photoactivation of alkyl C-H and silanization: a simple and general route to prepare high-density primary amines on inert polymer surfaces for protein immobilization. Biomacromolecules. 2009;10(5):1238–43.
Ellinger I, Schwab M, Stefanescu A, Hunziker W, Fuchs R. IgG transport across trophoblast-derived BeWo cells: a model system to study IgG transport in the placenta. Eur J Immunol. 1999;29(3):733–44.
Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, et al. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104(7):903–11.
Stefaner I, Praetor A, Hunziker W. Nonvectorial surface transport, endocytosis via a Di-leucine-based motif, and bidirectional transcytosis of chimera encoding the cytosolic tail of rat FcRn expressed in Madin-Darby canine kidney cells. J Biol Chem. 1999;274(13):8998–9005.
Raghavan M, Bonagura VR, Morrison SL, Bjorkman PJ. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry. 1995;34(45):14649–57.
Medesan C, Matesoi D, Radu C, Ghetie V, Ward ES. Delineation of the amino acid residues involved in transcytosis and catabolism of mouse IgG1. J Immunol. 1997;158(5):2211–7.
Tesar DB, Tiangco NE, Bjorkman PJ. Ligand valency affects transcytosis, recycling and intracellular trafficking mediated by the neonatal Fc receptor. Traffic. 2006;7(9):1127–42.
Gurbaxani BM, Morrison SL. Development of new models for the analysis of Fc-FcRn interactions. Mol Immunol. 2006;43(9):1379–89.
Gurbaxani B, Dela Cruz LL, Chintalacharuvu K, Morrison SL. Analysis of a family of antibodies with different half-lives in mice fails to find a correlation between affinity for FcRn and serum half-life. Mol Immunol. 2006;43(9):1462–73.
McGarry T, Hough R, Rogers S, Rechsteiner M. Intracellular distribution and degradation of immunoglobulin G and immunoglobulin G fragments injected into HeLa cells. J Cell Biol. 1983;96(2):338–46.
Ghetie V, Ward ES. Transcytosis and catabolism of antibody. Immunol Res. 2002;25(2):97–113.
Hershko A, Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–64.
Ghetie V, Popov S, Borvak J, Radu C, Matesoi D, Medesan C, et al. Increasing the serum persistence of an IgG fragment by random mutagenesis. Nat Biotechnol. 1997;15(7):637–40.
Dall’Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, et al. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J Immunol. 2002;169(9):5171–80.
Wang W, Lu P, Fang Y, Hamuro L, Pittman T, Carr B, et al. Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metab Dispos. 2011;39(9):1469–77.
Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol. 2007;179(7):4580–8.
Borvak J, Richardson J, Medesan C, Antohe F, Radu C, Simionescu M, et al. Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol. 1998;10(9):1289–98.
Zheng Y, Scheerens H, Davis JC Jr, Deng R, Fischer SK, Woods C, et al. Translational pharmacokinetics and pharmacodynamics of an FcRn-variant anti-CD4 monoclonal antibody from preclinical model to phase I study. Clin Pharmacol Ther. 2011;89(2):283–90.
Zia-Amirhosseini P, Minthorn E, Benincosa LJ, Hart TK, Hottenstein CS, Tobia LA, et al. Pharmacokinetics and pharmacodynamics of SB-240563, a humanized monoclonal antibody directed to human interleukin-5, in monkeys. J Pharmacol Exp Ther. 1999;291(3):1060–7.
Koon HB, Severy P, Hagg DS, Butler K, Hill T, Jones AG, et al. Antileukemic effect of daclizumab in CD25 high-expressing leukemias and impact of tumor burden on antibody dosing. Leuk Res. 2006;30(2):190–203.
Ng CM, Stefanich E, Anand BS, Fielder PJ, Vaickus L. Pharmacokinetics/pharmacodynamics of nondepleting anti-CD4 monoclonal antibody (TRX1) in healthy human volunteers. Pharm Res. 2006;23(1):95–103.
Berinstein NL, Grillo-Lopez AJ, White CA, Bence-Bruckler I, Maloney D, Czuczman M, et al. Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1998;9(9):995–1001.
Li J, Levi M, Charoin JE, et al. Rituximab exhibits a long half-life based on a population pharmacokinetic analysis in non-Hodgkin’s lymphoma (NHL) patients [abstract no. 2371]. Blood (ASH Annual Meeting Abstracts). 2007;110:700.
Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, et al. Predicting new molecular targets for known drugs. Nature. 2009;462(7270):175–81.
Rix U, Hantschel O, Durnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110(12):4055–63.
Hopkins AL. Network pharmacology. Nat Biotechnol. 2007;25(10):1110–1.
Deng R, Jin F, Prabhu S, Iyer S. Monoclonal antibodies: what are the pharmacokinetic and pharmacodynamic considerations for drug development? Expert Opin Drug Metab Toxicol. 2012;8(2):141–60.
Presta LG. Molecular engineering and design of therapeutic antibodies. Curr Opin Immunol. 2008;20(4):460–70.
Igawa T, Tsunoda H, Kuramochi T, Sampei Z, Ishii S, Hattori K. Engineering the variable region of therapeutic IgG antibodies. MAbs. 2011;3(3):243–52.
Waldrep JC, Noe RL, Stulting RD. Analysis of human corneal IgG by isoelectric focusing. Invest Ophthalmol Vis Sci. 1988;29(10):1538–43.
Boswell CA, Tesar DB, Mukhyala K, Theil FP, Fielder PJ, Khawli LA. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjug Chem. 2010;21(12):2153–63.
Khawli LA, Goswami S, Hutchinson R, Kwong ZW, Yang J, Wang X, et al. Charge variants in IgG1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats. MAbs. 2010;2(6):613–24.
Igawa T, Tsunoda H, Tachibana T, Maeda A, Mimoto F, Moriyama C, et al. Reduced elimination of IgG antibodies by engineering the variable region. Protein Eng Des Sel. 2010;23(5):385–92.
Dwek RA. Biological importance of glycosylation. Dev Biol Stand. 1998;96:43–7.
Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50.
Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther. 2007;7(9):1401–13.
Newkirk MM, Novick J, Stevenson MM, Fournier MJ, Apostolakos P. Differential clearance of glycoforms of IgG in normal and autoimmune-prone mice. Clin Exp Immunol. 1996;106(2):259–64.
Huang L, Biolsi S, Bales KR, Kuchibhotla U. Impact of variable domain glycosylation on antibody clearance: an LC/MS characterization. Anal Biochem. 2006;349(2):197–207.
Igawa T, Ishii S, Tachibana T, Maeda A, Higuchi Y, Shimaoka S, et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol. 2010;28(11):1203–7.
Finkelman FD, Madden KB, Morris SC, Holmes JM, Boiani N, Katona IM, et al. Anti-cytokine antibodies as carrier proteins: prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993;151(3):1235–44.
Faulstich H, Kirchner K, Derenzini M. Strongly enhanced toxicity of the mushroom toxin alpha-amanitin by an amatoxin-specific Fab or monoclonal antibody. Toxicon. 1988;26(5):491–9.
Fisher CJ Jr, Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein: the Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334(26):1697–702.
Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117(2):244–79.
Korth-Bradley JM, Rubin AS, Hanna RK, Simcoe DK, Lebsack ME. The pharmacokinetics of etanercept in healthy volunteers. Ann Pharmacother. 2000;34(2):161–4.
Kremer JM, Spencer-Green GT, Hanna RK, Korth-Bradley JM. Enbrel (Etanercept) pharmacokinetics in patient with rheumatoid arthritis [abstract]. Arthritis Rheum. 2000;43(Suppl):976.
Lon HK, Liu D, Zhang Q, DuBois DC, Almon RR, Jusko WJ. Pharmacokinetic-pharmacodynamic disease progression model for effect of etanercept in Lewis rats with collagen-induced arthritis. Pharm Res. 2011;28(7):1622–30.
Granneman RG, Zhang Y, Noertersheuser PA, Velagapudi RB, Awni WM, Locke CS. Pharmacokinetic/pharmacodynamic (PK/PD) relationships of adalimumab (HumiraTM) in rheumatoid arthritis (RA) patients during phase II/III clinical trials. Arthritis Rheum. 2003;48(Suppl):S140.
Zhu YW, Pendley C, Sisco D, Westhovens R, Durez P, Bouman-Thio E, et al. Pharmacokinetics and pharmacodynamics of infliximab, an anti-tumor necrosis factor-alpha monoclonal antibody, following single subcutaneous administrations in rheumatoid arthritis patients. Clin Pharmacol Ther. 2005;77:P43.
Hamilton RG, MacGlashan DW Jr, Saini SS. IgE antibody-specific activity in human allergic disease. Immunol Res. 2010;47(1–3):273–84.
Meno-Tetang GM, Lowe PJ. On the prediction of the human response: a recycled mechanistic pharmacokinetic/pharmacodynamic approach. Basic Clin Pharmacol Toxicol. 2005;96(3):182–92.
Hayashi N, Tsukamoto Y, Sallas WM, Lowe PJ. A mechanism-based binding model for the population pharmacokinetics and pharmacodynamics of omalizumab. Br J Clin Pharmacol. 2007;63(5):548–61.
Lowe PJ, Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol. 2011;72(2):306–20.
Putnam WS, Li J, Haggstrom J, Ng C, Kadkhodayan-Fischer S, Cheu M, et al. Use of quantitative pharmacology in the development of HAE1, a high-affinity anti-IgE monoclonal antibody. AAPS J. 2008;10(2):425–30.
Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368–80.
Woo S, Sun H, Pisle S, Figg WD. PK/PD modeling of anti-tumor effects of bevacizumab and thalidomide in combination with cytotoxic chemotherapeutic agent docetaxel in prostate cancer xenograft mouse model [abstract]. 2008 AAPS Annual Meeting and Exposition; Atlanta; 11–15 Nov 2008.
Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007;114(5):855–9.
Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46(2):726–33.
Anderson ME, Siahaan TJ. Targeting ICAM-1/LFA-1 interaction for controlling autoimmune diseases: designing peptide and small molecule inhibitors. Peptides. 2003;24(3):487–501.
Nast A, Kopp IB, Augustin M, Banditt KB, Boehncke WH, Follmann M, et al. Evidence-based (S3) guidelines for the treatment of psoriasis vulgaris. J Dtsch Dermatol Ges. 2007;5(Suppl 3):1–119.
Bauer RJ, Dedrick RL, White ML, Murray MJ, Garovoy MR. Population pharmacokinetics and pharmacodynamics of the anti-CD11a antibody hu1124 in human subjects with psoriasis. J Pharmacokinet Biopharm. 1999;27(4):397–420.
Ng CM, Joshi A, Dedrick RL, Garovoy MR, Bauer RJ. Pharmacokinetic-pharmacodynamic-efficacy analysis of efalizumab in patients with moderate to severe psoriasis. Pharm Res. 2005;22(7):1088–100.
Levi M, Grange S, Frey N. Exposure-response relationship of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in a large population of patients with rheumatoid arthritis. J Clin Pharmacol. Epub 2012 Feb 14.
Gibianski L, Frey N. Mechanistic modeling of the link between interleukin-6 receptor blockade with tocilizumab and its hematological effects [poster no. II-24]. Population Approach Group in Europe (PAGE); 7–10 Jun 2011; Athens. http://www.page-meeting.org/pdf_assets/2972-PAGE_2011_Poster_1965.pdf. Accessed 6 Nov 2012.
Ferrajoli A, O’Brien S, Keating MJ. Alemtuzumab: a novel monoclonal antibody. Expert Opin Biol Ther. 2001;1(6):1059–65.
Leget GA, Czuczman MS. Use of rituximab, the new FDA-approved antibody. Curr Opin Oncol. 1998;10(6):548–51.
Presta LG, Shields RL, Namenuk AK, Hong K, Meng YG. Engineering therapeutic antibodies for improved function. Biochem Soc Trans. 2002;30(4):487–90.
Todd PA, Brogden RN. Muromonab CD3: a review of its pharmacology and therapeutic potential. Drugs. 1989;37(6):871–99.
Janeway CA, Travers P, Walport M, Shlomchik J. Immunology. New York: Garland Publishing; 2001.
Zhou X, Hu W, Qin X. The role of complement in the mechanism of action of rituximab for B-cell lymphoma: implications for therapy. Oncologist. 2008;13(9):954–66.
Cohenuram M, Saif MW. Panitumumab the first fully human monoclonal antibody: from the bench to the clinic. Anticancer Drugs. 2007;18(1):7–15.
Ritter CA, Arteaga CL. The epidermal growth factor receptor-tyrosine kinase: a promising therapeutic target in solid tumors. Semin Oncol. 2003;30(1 Suppl 1):3–11.
Baselga J, Schöffski P, Rojo F, Dumez H, Ramos FJ, Macarulla T, et al. A phase I pharmacokinetic (PK) and molecular pharmacodynamic (PD) study of the combination of two anti-EGFR therapies, the monoclonal antibody (MAb) cetuximab (C) and the tyrosine kinase inhibitor (TKI) gefitinib (G), in patients (pts) with advanced colorectal (CRC), head and neck (HNC) and non-small cell lung cancer (NSCLC). J Clin Oncol. 2006;24(Suppl):3006.
Matar P, Rojo F, Cassia R, Moreno-Bueno G, Di Cosimo S, Tabernero J, et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer Res. 2004;10(19):6487–501.
Luo FR, Yang Z, Dong H, Camuso A, McGlinchey K, Fager K, et al. Correlation of pharmacokinetics with the antitumor activity of Cetuximab in nude mice bearing the GEO human colon carcinoma xenograft. Cancer Chemother Pharmacol. 2005;56(5):455–64.
Leonard DS, Hill AD, Kelly L, Dijkstra B, McDermott E, O’Higgins NJ. Anti-human epidermal growth factor receptor 2 monoclonal antibody therapy for breast cancer. Br J Surg. 2002;89(3):262–71.
Cuello M, Ettenberg SA, Clark AS, Keane MM, Posner RH, Nau MM, et al. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res. 2001;61(12):4892–900.
Junttila TT, Akita RW, Parsons K, Fields C. Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3 K complex is disrupted by trastuzumab and is effectively inhibited by the PI3 K inhibitor GDC-0941. Cancer Cell. 2009;15(5):429–40.
Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435–45.
Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–6.
Pfreundschuh M, Ho AD, Cavallin-Stahl E, Wolf M, Pettengell R, Vasova I, et al. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: an exploratory analysis of the MabThera International Trial Group (MInT) study. Lancet Oncol. 2008;9(5):435–44.
Ternant D, Cartron G, Hénin E, Tod M, Girard P, Paintaud G. Model-based design of rituximab dosage optimisation in follicular non-Hodgkin’s lymphoma. Br J Clin Pharmacol. 2012;73(4):597–605.
Mason U, Aldrich J, Breedveld F, Davis CB, Elliott M, Jackson M, et al. CD4 coating, but not CD4 depletion, is a predictor of efficacy with primatized monoclonal anti-CD4 treatment of active rheumatoid arthritis. J Rheumatol. 2002;29(2):220–9.
Hepburn TW, Totoritis MC, Davis CB. Antibody-mediated stripping of CD4 from lymphocyte cell surface in patients with rheumatoid arthritis. Rheumatology (Oxford). 2003;42(1):54–61.
Mould DR, Davis CB, Minthorn EA, Kwok DC, Elliott MJ, Luggen ME, et al. A population pharmacokinetic-pharmacodynamic analysis of single doses of clenoliximab in patients with rheumatoid arthritis. Clin Pharmacol Ther. 1999;66(3):246–57.
Sharma A, Davis CB, Tobia LA, Kwok DC, Tucci MG, Gore ER, et al. Comparative pharmacodynamics of keliximab and clenoliximab in transgenic mice bearing human CD4. J Pharmacol Exp Ther. 2000;293(1):33–41.
Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant. 2008;8(2):307–16.
Bluestone JA. CTLA-4Ig is finally making it: a personal perspective. Am J Transplant. 2005;5(3):423–4.
Kovarik J, Wolf P, Cisterne JM, Mourad G, Lebranchu Y, Lang P, et al. Disposition of basiliximab, an interleukin-2 receptor monoclonal antibody, in recipients of mismatched cadaver renal allografts. Transplantation. 1997;64(12):1701–5.
Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation: Daclizumab Triple Therapy Study Group. N Engl J Med. 1998;338(3):161–5.
Haba T, Uchida K, Katayama A, Tominaga Y, Sato T, Watanabe I, et al. Pharmacokinetics and pharmacodynamics of a chimeric interleukin-2 receptor monoclonal antibody, basiliximab, in renal transplantation: a comparison between Japanese and non-Japanese patients. Transplant Proc. 2001;33(7-8):3174–5.
Nagai T, Gotoh Y, Watarai Y, Tajima T, Arai K, Uchida K. Pharmacokinetics and pharmacodynamics of basiliximab in Japanese pediatric renal transplant patients. Int J Clin Pharmacol Ther. 2010;48(3):214–23.
Koch M, Niemeyer G, Patel I, Light S, Nashan B. Pharmacokinetics, pharmacodynamics, and immunodynamics of daclizumab in a two-dose regimen in liver transplantation. Transplantation. 2002;73(10):1640–6.
Kovarik J, Breidenbach T, Gerbeau C, Korn A, Schmidt AG, Nashan B. Disposition and immunodynamics of basiliximab in liver allograft recipients. Clin Pharmacol Ther. 1998;64(1):66–72.
Kovarik JM, Nashan B, Neuhaus P, Clavien PA, Gerbeau C, Hall ML, et al. A population pharmacokinetic screen to identify demographic-clinical covariates of basiliximab in liver transplantation. Clin Pharmacol Ther. 2001;69(4):201–9.
Genetta TB, Mauro VF. ABCIXIMAB: a new antiaggregant used in angioplasty. Ann Pharmacother. 1996;30(3):251–7.
Coller BS. Platelet GPIIb/IIIa antagonists: the first anti-integrin receptor therapeutics. J Clin Invest. 1997;100(11 Suppl):S57–60.
Scarborough RM, Kleiman NS, Phillips DR. Platelet glycoprotein IIb/IIIa antagonists: what are the relevant issues concerning their pharmacology and clinical use? Circulation. 1999;100(4):437–44.
Mager DE, Mascelli MA, Kleiman NS, Fitzgerald DJ, Abernethy DR. Simultaneous modeling of abciximab plasma concentrations and ex vivo pharmacodynamics in patients undergoing coronary angioplasty. J Pharmacol Exp Ther. 2003;307(3):969–76.
Trail PA, Bianchi AB. Monoclonal antibody drug conjugates in the treatment of cancer. Curr Opin Immunol. 1999;11(5):584–8.
Heath TD, Montgomery JA, Piper JR, Papahadjopoulos D. Antibody-targeted liposomes: increase in specific toxicity of methotrexate-gamma-aspartate. Proc Natl Acad Sci USA. 1983;80(5):1377–81.
Springer CJ, Bagshawe KD, Sharma SK, Searle F, Boden JA, Antoniw P, et al. Ablation of human choriocarcinoma xenografts in nude mice by antibody-directed enzyme prodrug therapy (ADEPT) with three novel compounds. Eur J Cancer. 1991;27(11):1361–6.
Bagshawe KD, Sharma SK, Springer CJ, Rogers GT. Antibody directed enzyme prodrug therapy (ADEPT): a review of some theoretical, experimental and clinical aspects. Ann Oncol. 1994;5(10):879–91.
Jain KK. Editorial: targeted drug delivery for cancer. Technol Cancer Res Treat. 2005;4(4):311–3.
Govindan SV, Griffiths GL, Hansen HJ, Horak ID, Goldenberg DM. Cancer therapy with radiolabeled and drug/toxin-conjugated antibodies. Technol Cancer Res Treat. 2005;4(4):375–91.
Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990;50(3 Suppl):814s–9s.
Fujimori K, Covell DG, Fletcher JE, Weinstein JN. Modeling analysis of the global and microscopic distribution of immunoglobulin G, F(ab′)2, and Fab in tumors. Cancer Res. 1989;49(20):5656–63.
Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, et al. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 1992;52(19):5144–53.
Witzig TE, White CA, Wiseman GA, Gordon LI, Emmanouilides C, Raubitschek A, et al. Phase I/II trial of IDEC-Y2B8 radioimmunotherapy for treatment of relapsed or refractory CD20(+) B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 1999;17(12):3793–803.
Friedberg JW, Fisher RI. Iodine-131 tositumomab (Bexxar): radioimmunoconjugate therapy for indolent and transformed B-cell non-Hodgkin’s lymphoma. Expert Rev Anticancer Ther. 2004;4(1):18–26.
van Dijk J, Zegveld ST, Fleuren GJ, Warnaar SO. Localization of monoclonal antibody G250 and bispecific monoclonal antibody CD3/G250 in human renal-cell carcinoma xenografts: relative effects of size and affinity. Int J Cancer. 1991;48(5):738–43.
Buchegger F, Pelegrin A, Delaloye B, Bischof-Delaloye A, Mach JP. Iodine-131-labeled MAb F(ab′)2 fragments are more efficient and less toxic than intact anti-CEA antibodies in radioimmunotherapy of large human colon carcinoma grafted in nude mice. J Nucl Med. 1990;31(6):1035–44.
Colapinto EV, Humphrey PA, Zalutsky MR, Groothuis DR, Friedman HS, de Tribolet N, et al. Comparative localization of murine monoclonal antibody Me1-14 F(ab′)2 fragment and whole IgG2a in human glioma xenografts. Cancer Res. 1988;48(20):5701–7.
Endo K, Kamma H, Ogata T. Radiolabeled monoclonal antibody 15 and its fragments for localization and imaging of xenografts of human lung cancer. J Natl Cancer Inst. 1988;80(11):835–42.
Harwood PJ, Boden J, Pedley RB, Rawlins G, Rogers GT, Bagshawe KD. Comparative tumour localization of antibody fragments and intact IgG in nude mice bearing a CEA-producing human colon tumour xenograft. Eur J Cancer Clin Oncol. 1985;21(12):1515–22.
Hendrix PG, Dauwe SE, Van De Voorde A, Nouwen EJ, Hoylaerts MF, De Broe ME. Radiolocalisation and imaging of stably HPLAP-transfected MO4 tumours with monoclonal antibodies and fragments. Br J Cancer. 1991;64(6):1060–8.
Milenic DE, Yokota T, Filpula DR, Finkelman MA, Dodd SW, Wood JF, et al. Construction, binding properties, metabolism, and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Res. 1991;51(23 Pt 1):6363–71.
Pedley RB, Boden JA, Boden R, Dale R, Begent RH. Comparative radioimmunotherapy using intact or F(ab′)2 fragments of 131I anti-CEA antibody in a colonic xenograft model. Br J Cancer. 1993;68(1):69–73.
Zhu H, Baxter LT, Jain RK. Potential and limitations of radioimmunodetection and radioimmunotherapy with monoclonal antibodies. J Nucl Med. 1997;38(5):731–41.
Zhu H, Jain RK, Baxter LT. Tumor pretargeting for radioimmunodetection and radioimmunotherapy. J Nucl Med. 1998;39(1):65–76.
Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19(13):3244–54.
Golay J, Di Gaetano N, Amico D, Cittera E, Barbui AM, Giavazzi R, et al. Gemtuzumab ozogamicin (Mylotarg) has therapeutic activity against CD33 acute lymphoblastic leukaemias in vitro and in vivo. Br J Haematol. 2005;128(3):310–7.
Jager E, van der Velden VH, te Marvelde JG, Walter RB, Agur Z, Vainstein V. Targeted drug delivery by gemtuzumab ozogamicin: mechanism-based mathematical model for treatment strategy improvement and therapy individualization. PLoS One. 2011;6(9):e24265.
Mathew J, Perez EA. Trastuzumab emtansine in human epidermal growth factor receptor 2-positive breast cancer: a review. Curr Opin Oncol. 2011;23(6):594–600.
Jumbe NL, Xin Y, Leipold DD, Crocker L, Dugger D, Mai E, et al. Modeling the efficacy of trastuzumab-DM1, an antibody drug conjugate, in mice. J Pharmacokinet Pharmacodyn. 2010;37(3):221–42.
Lee JJ, Chu E. An update on treatment advances for the first-line therapy of metastatic colorectal cancer. Cancer J. 2007;13(5):276–81.
Doppalapudi VR, Huang J, Liu D, Jin P, Liu B, Li L, et al. Chemical generation of bispecific antibodies. Proc Natl Acad Sci USA. 2010;107(52):22611–6.
Robinson MK, Hodge KM, Horak E, Sundberg AL, Russeva M, Shaller CC, et al. Targeting ErbB2 and ErbB3 with a bispecific single-chain Fv enhances targeting selectivity and induces a therapeutic effect in vitro. Br J Cancer. 2008;99(9):1415–25.
Shen J, Vil MD, Prewett M, Damoci C, Zhang H, Li H, et al. Development of a fully human anti-PDGFRbeta antibody that suppresses growth of human tumor xenografts and enhances antitumor activity of an anti-VEGFR2 antibody. Neoplasia. 2009;11(6):594–604.
Kontermann RE. Bispecific antibodies: developments and current perspectives. Berlin: Springer; 2011.
Park JW, Hong K, Kirpotin DB, Papahadjopoulos D, Benz CC. Immunoliposomes for cancer treatment. Adv Pharmacol. 1997;40:399–435.
Schnyder A, Huwyler J. Drug transport to brain with targeted liposomes. NeuroRx. 2005;2(1):99–107.
Cerletti A, Drewe J, Fricker G, Eberle AN, Huwyler J. Endocytosis and transcytosis of an immunoliposome-based brain drug delivery system. J Drug Target. 2000;8(6):435–46.
Shi N, Pardridge WM. Noninvasive gene targeting to the brain. Proc Natl Acad Sci USA. 2000;97(13):7567–72.
Shi N, Zhang Y, Zhu C, Boado RJ, Pardridge WM. Brain-specific expression of an exogenous gene after i.v. administration. Proc Natl Acad Sci USA. 2001;98(22):12754–9.
Zhang Y, Calon F, Zhu C, Boado RJ, Pardridge WM. Intravenous nonviral gene therapy causes normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism. Hum Gene Ther. 2003;14(1):1–12.
Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, et al. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8(4):1172–81.
Matsumura Y, Gotoh M, Muro K, Yamada Y, Shirao K, Shimada Y, et al. Phase I and pharmacokinetic study of MCC-465, a doxorubicin (DXR) encapsulated in PEG immunoliposome, in patients with metastatic stomach cancer. Ann Oncol. 2004;15(3):517–25.
Lee HJ, Zhang Y, Zhu C, Duff K, Pardridge WM. Imaging brain amyloid of Alzheimer disease in vivo in transgenic mice with an Abeta peptide radiopharmaceutical. J Cereb Blood Flow Metab. 2002;22(2):223–31.
Bulte JW, Douglas T, Mann S, Frankel RB, Moskowitz BM, Brooks RA, et al. Magnetoferritin: characterization of a novel superparamagnetic MR contrast agent. J Magn Reson Imaging. 1994;4(3):497–505.
Kurihara A, Pardridge WM. Aβ1−40 peptide radiopharmaceuticals for brain amyloid imaging: 111In chelation, conjugation to poly(ethylene glycol)-biotin linkers, and autoradiography with Alzheimer’s disease brain sections. Bioconjug Chem. 2000;11(3):380–6.
Herrlinger U, Kramm CM, Aboody-Guterman KS, Silver JS, Ikeda K, Johnston KM, et al. Pre-existing herpes simplex virus 1 (HSV-1) immunity decreases, but does not abolish, gene transfer to experimental brain tumors by a HSV-1 vector. Gene Ther. 1998;5(6):809–19.
Kajiwara K, Byrnes AP, Ohmoto Y, Charlton HM, Wood MJ, Wood KJ. Humoral immune responses to adenovirus vectors in the brain. J Neuroimmunol. 2000;103(1):8–15.
Radler JO, Koltover I, Salditt T, Safinya CR. Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science. 1997;275(5301):810–4.
Bagshawe KD. Antibody-directed enzyme prodrug therapy (ADEPT) for cancer. Expert Rev Anticancer Ther. 2006;6(10):1421–31.
Napier MP, Sharma SK, Springer CJ, Bagshawe KD, Green AJ, Martin J, et al. Antibody-directed enzyme prodrug therapy: efficacy and mechanism of action in colorectal carcinoma. Clin Cancer Res. 2000;6(3):765–72.
Bagshawe KD. Antibody-directed enzyme prodrug therapy for cancer: its theoretical basis and application. Mol Med Today. 1995;1(9):424–31.
Springer CJ, Poon GK, Sharma SK, Bagshawe KD. Identification of prodrug, active drug, and metabolites in an ADEPT clinical study. Cell Biophys. 1993;22(1–3):9–26.
Martin J, Stribbling SM, Poon GK, Begent RH, Napier M, Sharma SK, et al. Antibody-directed enzyme prodrug therapy: pharmacokinetics and plasma levels of prodrug and drug in a phase I clinical trial. Cancer Chemother Pharmacol. 1997;40(3):189–201.
Medzihradszky KF, Spencer DI, Sharma SK, Bhatia J, Pedley RB, Read DA, et al. Glycoforms obtained by expression in Pichia pastoris improve cancer targeting potential of a recombinant antibody-enzyme fusion protein. Glycobiology. 2004;14(1):27–37.
Getmanova EV, Chen Y, Bloom L, Gokemeijer J, Shamah S, Warikoo V, et al. Antagonists to human and mouse vascular endothelial growth factor receptor 2 generated by directed protein evolution in vitro. Chem Biol. 2006;13(5):549–56.
Silverman J, Liu Q, Bakker A, To W, Duguay A, Alba BM, et al. Multivalent avimer proteins evolved by exon shuffling of a family of human receptor domains. Nat Biotechnol. 2005;23(12):1556–61.
Binz HK, Stumpp MT, Forrer P, Amstutz P, Pluckthun A. Designing repeat proteins: well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins. J Mol Biol. 2003;332(2):489–503.
Hey T, Fiedler E, Rudolph R, Fiedler M. Artificial, non-antibody binding proteins for pharmaceutical and industrial applications. Trends Biotechnol. 2005;23(10):514–22.
Wheeler YY, Chen SY, Sane DC. Intrabody and intrakine strategies for molecular therapy. Mol Ther. 2003;8(3):355–66.
Boldicke T, Weber H, Mueller PP, Barleon B, Bernal M. Novel highly efficient intrabody mediates complete inhibition of cell surface expression of the human vascular endothelial growth factor receptor-2 (VEGFR-2/KDR). J Immunol Methods. 2005;300(1–2):146–59.
Shin I, Edl J, Biswas S, Lin PC, Mernaugh R, Arteaga CL. Proapoptotic activity of cell-permeable anti-Akt single-chain antibodies. Cancer Res. 2005;65(7):2815–24.
Strube RW, Chen SY. Characterization of anti-cyclin E single-chain Fv antibodies and intrabodies in breast cancer cells: enhanced intracellular stability of novel sFv-F(c) intrabodies. J Immunol Method. 2002;263(1–2):149–67.
Williams BR, Zhu Z. Intrabody-based approaches to cancer therapy: status and prospects. Curr Med Chem. 2006;13(12):1473–80.
Marasco WA, LaVecchio J, Winkler A. Human anti-HIV-1 tat sFv intrabodies for gene therapy of advanced HIV-1-infection and AIDS. J Immunol Method. 1999;231(1–2):223–38.
Doorbar J, Griffin H. Intrabody strategies for the treatment of human papillomavirus-associated disease. Expert Opin Biol Ther. 2007;7(5):677–89.
Messer A, Lynch SM, Butler DC. Developing intrabodies for the therapeutic suppression of neurodegenerative pathology. Expert Opin Biol Ther. 2009;9(9):1189–97.
Morgan ET, Goralski KB, Piquette-Miller M, Renton KW, Robertson GR, Chaluvadi MR, et al. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos. 2008;36(2):205–16.
Morgan ET. Impact of infectious and inflammatory disease on cytochrome P450-mediated drug metabolism and pharmacokinetics. Clin Pharmacol Ther. 2009;85(4):434–8.
Prandota J. Important role of proinflammatory cytokines/other endogenous substances in drug-induced hepatotoxicity: depression of drug metabolism during infections/inflammation states, and genetic polymorphisms of drug-metabolizing enzymes/cytokines may markedly contribute to this pathology. Am J Ther. 2005;12(3):254–61.
Abdel-Razzak Z, Loyer P, Fautrel A, Gautier JC, Corcos L, Turlin B, et al. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol. 1993;44(4):707–15.
Aitken AE, Morgan ET. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos. 2007;35(9):1687–93.
Schmitt C, Kuhn B, Zhang X, Kivitz AJ, Grange S. Disease-drug-drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2011;89(5):735–40.
Strehlau J, Pape L, Offner G, Nashan B, Ehrich JH. Interleukin-2 receptor antibody-induced alterations of ciclosporin dose requirements in paediatric transplant recipients. Lancet. 2000;356(9238):1327–8.
Vasquez EM, Pollak R. OKT3 therapy increases cyclosporine blood levels. Clin Transplant. 1997;11(1):38–41.
Sifontis NM, Benedetti E, Vasquez EM. Clinically significant drug interaction between basiliximab and tacrolimus in renal transplant recipients. Transplant Proc. 2002;34(5):1730–2.
Huang SM, Zhao H, Lee JI, Reynolds K, Zhang L, Temple R, et al. Therapeutic protein-drug interactions and implications for drug development. Clin Pharmacol Ther. 2010;87(4):497–503.
Dickmann LJ, Patel SK, Rock DA, Wienkers LC, Slatter JG. Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos. 2011;39(8):1415–22.
Dickmann LJ, Patel SK, Wienkers LC, Slatter JG. Effects of Interleukin 1beta (IL-1beta) and IL-1beta/Interleukin 6 (IL-6) combinations on drug metabolizing enzymes in human hepatocyte culture. Curr Drug Metab. 2012;13(7):930–7.
Donato MT, Guillen MI, Jover R, Castell JV, Gomez-Lechon MJ. Nitric oxide-mediated inhibition of cytochrome P450 by interferon-gamma in human hepatocytes. J Pharmacol Exp Ther. 1997;281(1):484–90.
Islam M, Frye RF, Richards TJ, Sbeitan I, Donnelly SS, Glue P, et al. Differential effect of IFNalpha-2b on the cytochrome P450 enzyme system: a potential basis of IFN toxicity and its modulation by other drugs. Clin Cancer Res. 2002;8(8):2480–7.
Lee CM, Pohl J, Morgan ET. Dual mechanisms of CYP3A protein regulation by proinflammatory cytokine stimulation in primary hepatocyte cultures. Drug Metab Dispos. 2009;37(4):865–72.
Molanaei H, Stenvinkel P, Qureshi AR, Carrero JJ, Heimburger O, Lindholm B, et al. Metabolism of alprazolam (a marker of CYP3A4) in hemodialysis patients with persistent inflammation. Eur J Clin Pharmacol. 2012;68(5):571–7.
Pascussi JM, Gerbal-Chaloin S, Pichard-Garcia L, Daujat M, Fabre JM, Maurel P, et al. Interleukin-6 negatively regulates the expression of pregnane X receptor and constitutively activated receptor in primary human hepatocytes. Biochem Biophys Res Commun. 2000;274(3):707–13.
Sunman JA, Hawke RL, LeCluyse EL, Kashuba AD. Kupffer cell-mediated IL-2 suppression of CYP3A activity in human hepatocytes. Drug Metab Dispos. 2004;32(3):359–63.
Yang Q, Doshi U, Li N, Li AP. Effects of culture duration on gene expression of p450 isoforms, uptake and efflux transporters in primary hepatocytes cultured in the absence and presence of interleukin-6: implications for experimental design for the evaluation of downregulatory effects of biotherapeutics. Curr Drug Metab. 2012;13(7):938–46.
Vee ML, Lecureur V, Stieger B, Fardel O. Regulation of drug transporter expression in human hepatocytes exposed to the proinflammatory cytokines tumor necrosis factor-alpha or interleukin-6. Drug Metab Dispos. 2009;37(3):685–93.
Teng S, Piquette-Miller M. The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther. 2005;312(2):841–8.
Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Reduction in cytochrome P-450 enzyme expression is associated with repression of CAR (constitutive androstane receptor) and PXR (pregnane X receptor) in mouse liver during the acute phase response. Biochem Biophys Res Commun. 2002;293(1):145–9.
Rostami-Hodjegan A, Tucker GT. Simulation and prediction of in vivo drug metabolism in human populations from in vitro data. Nat Rev Drug Discov. 2007;6(2):140–8.
Rostami-Hodjegan A, Tucker G. ‘In silico’ simulations to assess the ‘in vivo’ consequences of ‘in vitro’ metabolic drug–drug interactions. Drug Discov Today. 2004;1(4):441–8.
Dallas S, Sensenhauser C, Batheja A, Singer M, Markowska M, Zakszewski C, et al. De-risking bio-therapeutics for possible drug interactions using cryopreserved human hepatocytes. Curr Drug Metab. 2012;13(7):923–9.
Kraynov E, Martin SW, Hurst S, Fahmi OA, Dowty M, Cronenberger C, et al. How current understanding of clearance mechanisms and pharmacodynamics of therapeutic proteins can be applied for evaluation of their drug-drug interaction potential. Drug Metab Dispos. 2011;39(10):1779–83.
Zhou H, Davis HM. Risk-based strategy for the assessment of pharmacokinetic drug-drug interactions for therapeutic monoclonal antibodies. Drug Discov Today. 2009;14(17–18):891–8.
Hocker B, Kovarik JM, Daniel V, Opelz G, Fehrenbach H, Holder M, et al. Pharmacokinetics and immunodynamics of basiliximab in pediatric renal transplant recipients on mycophenolate mofetil comedication. Transplantation. 2008;86(9):1234–40.
Bunescu A, Seideman P, Lenkei R, Levin K, Egberg N. Enhanced Fcgamma receptor I, alphaMbeta2 integrin receptor expression by monocytes and neutrophils in rheumatoid arthritis: interaction with platelets. J Rheumatol. 2004;31(12):2347–55.
Sharpe AH, Abbas AK. T-cell costimulation–biology, therapeutic potential, and challenges. N Engl J Med. 2006;355(10):973–5.
Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355(10):1018–28.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92.
Henson ES, Hu X, Gibson SB. Herceptin sensitizes ErbB2-overexpressing cells to apoptosis by reducing antiapoptotic Mcl-1 expression. Clin Cancer Res. 2006;12(3 Pt 1):845–53.
Bruno R, Washington CB, Lu JF, Lieberman G, Banken L, Klein P. Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother Pharmacol. 2005;56(4):361–9.
Leyland-Jones B, Gelmon K, Ayoub JP, Arnold A, Verma S, Dias R, et al. Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol. 2003;21(21):3965–71.
Inoue K, Slaton JW, Perrotte P, Davis DW, Bruns CJ, Hicklin DJ, et al. Paclitaxel enhances the effects of the anti-epidermal growth factor receptor monoclonal antibody ImClone C225 in mice with metastatic human bladder transitional cell carcinoma. Clin Cancer Res. 2000;6(12):4874–84.
Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, et al. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II trial. J Clin Oncol. 2004;22(13):2610–6.
Dowlati A, Nethery D, Liu J. Combined inhibition of epidermal growth factor receptor (EGFR) and JAK/Stat signaling results in superior growth inhibition in A431 cell line as compared to single agent therapy [abstract]. Proc Am Assoc Cancer Res. 2003;44(800):459.
Finn RS, Wilson CA, Sanders J. Targeting the epidermal growth factor receptor (EGFR) and HER-2 with OSI-774 and trastuzumab, respectively, in HER-2 overexpressing human breast cancer cell lines results in a therapeutic advantage in vitro [abstract]. Proc Am Assoc Cancer Res. 2003;44:235.
Huang S, Armstrong E, Chinnaiyan P, Harari PM. Dual agent molecular targeting of the epidermal growth factor receptor: combining anti-HER1/EGFR monoclonal antibody with tyrosine kinase inhibitor [abstract]. Proc Am Assoc Cancer Res. 2003;44:3777.
Huang S, Armstrong EA, Benavente S, Chinnaiyan P, Harari PM. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004;64(15):5355–62.
Kauh JS, Laguinge L, Lin S, Jessup JM. Combined tyrosine kinase inhibition of c-erb B-2 and EGFR in pancreatic adenocarcinoma leads to increased inhibition of cell growth [abstract]. Proc Am Assoc Cancer Res. 2003;22:876.
Matar P, Rojo F, Cassia R, Moreno-Bueno G, Di Cosimo S, Tabernero J, et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer Res. 2004;10:6487–501.
Rose WC, Wild R. Therapeutic synergy of oral taxane BMS-275183 and cetuximab versus human tumor xenografts. Clin Cancer Res. 2004;10(21):7413–7.
Hallifax D, Houston JB. Evaluation of hepatic clearance prediction using in vitro data: emphasis on fraction unbound in plasma and drug ionisation using a database of 107 drugs. J Pharm Sci. 2012;101(8):2645–52.
Jamei M, Dickinson GL, Rostami-Hodjegan A. A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: a tale of ‘bottom-up’ vs ‘top-down’ recognition of covariates. Drug Metab Pharmacokinet. 2009;24(1):53–75.
Jones RD, Jones HM, Rowland M, Gibson CR, Yates JW, Chien JY, et al. PhRMA CPCDC initiative on predictive models of human pharmacokinetics, part 2: comparative assessment of prediction methods of human volume of distribution. J Pharm Sci. (epub 2011 Mar 30).
Poulin P, Jones HM, Jones RD, Yates JW, Gibson CR, Chien JY, et al. PhRMA CPCDC initiative on predictive models of human pharmacokinetics, part 1: goals, properties of the PhRMA dataset, and comparison with literature datasets. J Pharm Sci. (epub 2011 Apr 26).
Poulin P, Jones RD, Jones HM, Gibson CR, Rowland M, Chien JY, et al. PHRMA CPCDC initiative on predictive models of human pharmacokinetics, part 5: prediction of plasma concentration-time profiles in human by using the physiologically-based pharmacokinetic modeling approach. J Pharm Sci. (epub 2011 May 3).
Ring BJ, Chien JY, Adkison KK, Jones HM, Rowland M, Jones RD, et al. PhRMA CPCDC initiative on predictive models of human pharmacokinetics, part 3: comparative assessement of prediction methods of human clearance. J Pharm Sci. (epub 2011 May 3).
Huh Y, Smith DE, Feng MR. Interspecies scaling and prediction of human clearance: comparison of small- and macro-molecule drugs. Xenobiotica. 2011;41(11):972–87.
Mahmood I. Interspecies scaling of protein drugs: prediction of clearance from animals to humans. J Pharm Sci. 2004;93(1):177–85.
Mahmood I. Pharmacokinetic allometric scaling of antibodies: application to the first-in-human dose estimation. J Pharm Sci. 2009;98(10):3850–61.
Mordenti J, Chen SA, Moore JA, Ferraiolo BL, Green JD. Interspecies scaling of clearance and volume of distribution data for five therapeutic proteins. Pharm Res. 1991;8(11):1351–9.
Lin YS, Nguyen C, Mendoza JL, Escandon E, Fei D, Meng YG, et al. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther. 1999;288(1):371–8.
Bazin-Redureau M, Pepin S, Hong G, Debray M, Scherrmann JM. Interspecies scaling of clearance and volume of distribution for horse antivenom F(ab′)2. Toxicol Appl Pharmacol. 1998;150(2):295–300.
Grene-Lerouge NA, Bazin-Redureau MI, Debray M, Scherrmann JM. Interspecies scaling of clearance and volume of distribution for digoxin-specific Fab. Toxicol Appl Pharmacol. 1996;138(1):84–9.
Ling J, Zhou H, Jiao Q, Davis HM. Interspecies scaling of therapeutic monoclonal antibodies: initial look. J Clin Pharmacol. 2009;49(12):1382–402.
Wang W, Prueksaritanont T. Prediction of human clearance of therapeutic proteins: simple allometric scaling method revisited. Biopharm Drug Dispos. 2010;31(4):253–63.
Oitate M, Nakayama S, Ito T, Kurihara A, Okudaira N, Izumi T. Prediction of human plasma concentration-time profiles of monoclonal antibodies from monkey data by a species-invariant time method. Drug Metab Pharmacokinet. 2012;27(3):354–9.
Wajima T, Yano Y, Fukumura K, Oguma T. Prediction of human pharmacokinetic profile in animal scale up based on normalizing time course profiles. J Pharm Sci. 2004;93(7):1890–900.
Mahmood I, Goteti K. Prediction of drug concentration-time data in humans from animals: a comparison of three methods. Xenobiotica. 2012;42(8):756–65.
Mahmood I. Prediction of clearance and volume of distribution in the obese from normal weight subjects: an allometric approach. Clin Pharmacokinet. 2012;51(8):527–42.
Yates JW, Arundel PA. On the volume of distribution at steady state and its relationship with two-compartmental models. J Pharm Sci. 2008;97(1):111–22.
Straughn AB. Limitations of noncompartmental pharmacokinetic analysis of biotech drugs. In: Meibohm B, editor. Pharmacokinetics and pharmacodynamics of biotech drugs. Weinheim: Wiley; 2006. p. 181–8.
Xu Z, Seitz K, Fasanmade A, Ford J, Williamson P, Xu W, et al. Population pharmacokinetics of infliximab in patients with ankylosing spondylitis. J Clin Pharmacol. 2008;48(6):681–95.
Molthoff CF, Pinedo HM, Schluper HM, Nijman HW, Boven E. Comparison of the pharmacokinetics, biodistribution and dosimetry of monoclonal antibodies OC125, OV-TL 3, and 139H2 as IgG and F(ab′)2 fragments in experimental ovarian cancer. Br J Cancer. 1992;65(5):677–83.
Kairemo KJ, Lappalainen AK, Kaapa E, Laitinen OM, Hyytinen T, Karonen SL, et al. In vivo detection of intervertebral disk injury using a radiolabeled monoclonal antibody against keratan sulfate. J Nucl Med. 2001;42(3):476–82.
Danilov SM, Gavrilyuk VD, Franke FE, Pauls K, Harshaw DW, McDonald TD, et al. Lung uptake of antibodies to endothelial antigens: key determinants of vascular immunotargeting. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1335–47.
Levy G. Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther. 1994;56(3):248–52.
Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28(6):507–32.
Aston PJ, Derks G, Raji A, Agoram BM, van der Graaf PH. Mathematical analysis of the pharmacokinetic-pharmacodynamic (PKPD) behaviour of monoclonal antibodies: predicting in vivo potency. J Theor Biol. 2011;281(1):113–21.
Grimm HP. Gaining insights into the consequences of target-mediated drug disposition of monoclonal antibodies using quasi-steady-state approximations. J Pharmacokinet Pharmacodyn. 2009;36(5):407–20.
Betts AM, Clark TH, Yang J, Treadway JL, Li M, Giovanelli MA, et al. The application of target information and preclinical pharmacokinetic/pharmacodynamic modeling in predicting clinical doses of a Dickkopf-1 antibody for osteoporosis. J Pharmacol Exp Ther. 2010;333(1):2–13.
Luu KT, Bergqvist S, Chen E, Hu-Lowe D, Kraynov E. A model-based approach to predicting the human pharmacokinetics of a monoclonal antibody exhibiting target-mediated drug disposition. J Pharmacol Exp Ther. 2012;341(3):702–8.
Gibiansky L, Gibiansky E, Kakkar T, Ma P. Approximations of the target-mediated drug disposition model and identifiability of model parameters. J Pharmacokinet Pharmacodyn. 2008;35(5):573–91.
Mager DE, Krzyzanski W. Quasi-equilibrium pharmacokinetic model for drugs exhibiting target-mediated drug disposition. Pharm Res. 2005;22(10):1589–96.
Ma P. Theoretical considerations of target-mediated drug disposition models: simplifications and approximations. Pharm Res. 2012;29(3):866–82.
Peletier LA, Gabrielsson J. Dynamics of target-mediated drug disposition: characteristic profiles and parameter identification. J Pharmacokinet Pharmacodyn. 2012;39(5):429–51.
Yan X, Mager DE, Krzyzanski W. Selection between Michaelis-Menten and target-mediated drug disposition pharmacokinetic models. J Pharmacokinet Pharmacodyn. 2010;37(1):25–47.
Peletier LA, Gabrielsson J. Dynamics of target-mediated drug disposition. Eur J Pharm Sci. 2009;38(5):445–64.
ter Meulen CG, Goertz JH, Klasen IS, Verweij CM, Hilbrands LB, Wetzels JF, et al. Decreased renal excretion of soluble interleukin-2 receptor alpha after treatment with daclizumab. Kidney Int. 2003;64(2):697–703.
Gibiansky L, Gibiansky E. Target-mediated drug disposition model for drugs that bind to more than one target. J Pharmacokinet Pharmacodyn. 2010;37(4):323–46.
Nestorov I. Whole body pharmacokinetic models. Clin Pharmacokinet. 2003;42(10):883–908.
Edginton AN, Theil FP, Schmitt W, Willmann S. Whole body physiologically-based pharmacokinetic models: their use in clinical drug development. Expert Opin Drug Metab Toxicol. 2008;4(9):1143–52.
Thygesen P, Macheras P, Van Peer A. Physiologically-based PK/PD modelling of therapeutic macromolecules. Pharm Res. 2009;26(12):2543–50.
Shah DK, Betts AM. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn. 2012;39(1):67–86.
Covell DG, Barbet J, Holton OD, Black CD, Parker RJ, Weinstein JN. Pharmacokinetics of monoclonal immunoglobulin G1, F(ab′)2, and Fab′ in mice. Cancer Res. 1986;46(8):3969–78.
Rippe B, Haraldsson B. Fluid and protein fluxes across small and large pores in the microvasculature: application of two-pore equations. Acta Physiol Scand. 1987;131(3):411–28.
Heiskanen T, Kairemo K. Development of a PBPK model for monoclonal antibodies and simulation of human and mice PBPK of a radiolabelled monoclonal antibody. Curr Pharm Des. 2009;15(9):988–1007.
Fang L, Sun D. Predictive physiologically based pharmacokinetic model for antibody-directed enzyme prodrug therapy. Drug Metab Dispos. 2008;36(6):1153–65.
Hansen RJ, Balthasar JP. Pharmacokinetic/pharmacodynamic modeling of the effects of intravenous immunoglobulin on the disposition of antiplatelet antibodies in a rat model of immune thrombocytopenia. J Pharm Sci. 2003;92(6):1206–15.
Xiao JJ. Pharmacokinetic models for FcRn-mediated IgG disposition. J Biomed Biotechnol. 2012;2012:282989.
Chen Y, Balthasar JP. Evaluation of a catenary PBPK model for predicting the in vivo disposition of mAbs engineered for high-affinity binding to FcRn. AAPS J. 2012;14(4):850–9.
Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor. FcRn. J Immunol. 2004;172(4):2021–9.
Hopkins CR, Trowbridge IS. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983;97(2):508–21.
Vaughn DE, Bjorkman PJ. High-affinity binding of the neonatal Fc receptor to its IgG ligand requires receptor immobilization. Biochemistry. 1997;36(31):9374–80.
Yamashiro DJ, Maxfield FR. Kinetics of endosome acidification in mutant and wild-type Chinese hamster ovary cells. J Cell Biol. 1987;105(6 Pt 1):2713–21.
Davda JP, Jain M, Batra SK, Gwilt PR, Robinson DH. A physiologically based pharmacokinetic (PBPK) model to characterize and predict the disposition of monoclonal antibody CC49 and its single chain Fv constructs. Int Immunopharmacol. 2008;8(3):401–13.
Chabot JR, Dettling DE, Jasper PJ, Gomes BC. Comprehensive mechanism-based antibody pharmacokinetic modeling. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:4318–23.
Orencia® (abatacept). http://www.orencia.com/index.aspx. Accessed 22 Nov 2012.
Hasegawa M, Imai Y, Hiraoka M, Ito K, Roy A. Model-based determination of abatacept exposure in support of the recommended dose for Japanese rheumatoid arthritis patients. J Pharmacokinet Pharmacodyn. 2011;38(6):803–32.
European Medicines Agency. Assessment report for Humira. London, 24 July 2008. EMEA/CHMP/479654/2008. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000481/WC500050877.pdf. Accessed 22 Nov 2012.
Velagapudi R, Noertershauser P, Awni W, Granneman R, Kupper H. Bioavailability of adalimumab following subcutaneous injections in rheumatoid arthritis patients [abstract]. Clin Pharmacol Ther. 2005;77:P84.
Mould DR, Baumann A, Kuhlmann J, Keating MJ, Weitman S, Hillmen P, et al. Population pharmacokinetics-pharmacodynamics of alemtuzumab (Campath) in patients with chronic lymphocytic leukaemia and its link to treatment response. Br J Clin Pharmacol. 2007;64(3):278–91.
Mentre F, Kovarik J, Gerbeau C. Constructing a prediction interval for time to reach a threshold concentration based on a population pharmacokinetic analysis: an application to basiliximab in renal transplantation. J Pharmacokinet Biopharm. 1999;27(2):213–30.
Anhang I. Zusammenfassung der merkmale des arzneimittels. Benlysta 120 mg Pulver zur Herstellung eines Infusionslösungskonzentrats. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002015/WC500110152.pdf. Accessed 22 Nov 2012.
Anhang I. Zusammenfassung der merkmale des arzneimittels. Ilaris 150 mg Pulver zur Herstellung einer Injektionslösung. http:// www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001109/WC500031680.pdf. Accessed 22 Nov 2012.
Annex I: summary of product characteristics. Removab 10 microgram concentrate for solution for infusion. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000972/WC500051809.pdf. Accessed 22 Nov 2012.
Annex I: summary of product characteristics. Cimzia 200 mg solution for injection. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001037/WC500069763.pdf. Accessed 22 Nov 2012.
Dirks NL, Nolting A, Kovar A, Meibohm B. Population pharmacokinetics of cetuximab in patients with squamous cell carcinoma of the head and neck. J Clin Pharmacol. 2008;48(3):267–78.
Pescovitz MD, Bumgardner G, Gaston RS, Kirkman RL, Light S, Patel IH, et al. Pharmacokinetics of daclizumab and mycophenolate mofetil with cyclosporine and steroids in renal transplantation. Clin Transplant. 2003;17(6):511–7.
Marathe A, Peterson MC, Mager DE. Integrated cellular bone homeostasis model for denosumab pharmacodynamics in multiple myeloma patients. J Pharmacol Exp Ther. 2008;326(2):555–62.
Annex I: summary of product characteristics. Soliris 300 mg concentrate for solution for infusion. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000791/WC500054208.pdf. Accessed 22 Nov 2012.
Sun YN, Lu JF, Joshi A, Compton P, Kwon P, Bruno RA. Population pharmacokinetics of efalizumab (humanized monoclonal anti-CD11a antibody) following long-term subcutaneous weekly dosing in psoriasis subjects. J Clin Pharmacol. 2005;45(4):468–76.
Zhu Y, Zhou Q, Wang Y. Role of transforming growth factor beta3 on amylase secretion of submandibular gland cells in rat [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2004;18(6):490–3.
Zhou SY, Shu C, Korth-Bradley J, Raible D, Palmisano M, Wadjula J, et al. Integrated population pharmacokinetics of etanercept in healthy subjects and in patients with rheumatoid arthritis and ankylosing spondylitis. J Clin Pharmacol. 2011;51(6):864–75.
Dowell JA, King SP, Liu H, Berger MS, Korth-Bradley JM. A population pharmacokinetic analysis of a new antibode chemotherapeutic agent: gemtuzumab ozogamicin [abstract no. 116]. Annual Meeting of the Population Approach Group in Europe; 15–16 Jun 2000; Salamanca. p. 9. http://www.page-meeting.org/default.asp?abstract=116. Accessed 6 Nov 2012.
Zhou H, Jang H, Fleischmann RM, Bouman-Thio E, Xu Z, Marini JC, et al. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-alpha monoclonal antibody, in subjects with rheumatoid arthritis. J Clin Pharmacol. 2007;47(3):383–96.
Xu Z, Vu T, Lee H, Hu C, Ling J, Yan H, et al. Population pharmacokinetics of golimumab, an anti-tumor necrosis factor-alpha human monoclonal antibody, in patients with psoriatic arthritis. J Clin Pharmacol. 2009;49(9):1056–70.
Anhang I: zusammenfassung der merkmale des arzneimittels. Zevalin 1,6 mg/ml Kit für ein radioaktives Arzneimittel zur Infusion. http://www.ema.europa.eu/docs/de_DE/document_library/EPAR_-_Product_Information/human/000547/WC500049469.pdf. Accessed 22 Nov 2012.
Fasanmade AA, Adedokun OJ, Ford J, Hernandez D, Johanns J, Hu C, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol. 2009;65(12):1211–28.
Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13(6):1810–5.
Weiner LM. Fully human therapeutic monoclonal antibodies. J Immunother. 2006;29(1):1–9.
Anhang I. Zusammenfassung der merkmale des arzneimittels. TYSABRI 300 mg Konzentrat zur Herstellung einer Infusionslösung. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000603/WC500044688.pdf. Accessed 22 Nov 2012.
Coiffier B, Losic N, Ronn BB, Lepretre S, Pedersen LM, Gadeberg O, et al. Pharmacokinetics and pharmacokinetic/pharmacodynamic associations of ofatumumab, a human monoclonal CD20 antibody, in patients with relapsed or refractory chronic lymphocytic leukaemia: a phase 1–2 study. Br J Haematol. 2010;150(1):58–71.
Anhang I: zusammenfassung der merkmale des arzneimittels. Vectibix 20 mg/ml Konzentrat zur Herstellung einer Infusionslösung. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000741/WC500047710.pdf. Accessed 22 Nov 2012.
Ma P, Yang BB, Wang YM, Peterson M, Narayanan A, Sutjandra L, et al. Population pharmacokinetic analysis of panitumumab in patients with advanced solid tumors. J Clin Pharmacol. 2009;49(10):1142–56.
Busbee BG, Murahashi WY, Yao Z, Zhang Y. Ranibizumab pharmacokinetics in retinal vein occlusion [poster no. 4874]. Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO); 1–5 May 2011; Fort Lauderdale.
Ng CM, Bruno R, Combs D, Davies B. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol. 2005;45(7):792–801.
Frey N, Grange S, Woodworth T. Population pharmacokinetic analysis of tocilizumab in patients with rheumatoid arthritis. J Clin Pharmacol. 2010;50(7):754–66.
Bexxar (tositumomab and iodineI 131 tositumomab): highlights of prescribing information. http://us.gsk.com/products/assets/us_bexxar.pdf. Accessed 22 Nov 2012.
Fukushima Y, Charoin JE, Brewster M, Jonsson EN. Population pharmacokinetic analysis of trastuzumab (Herceptin®) based on data from three different dosing regimens [abstract no. 1121]. Sixteenth Meeting of the Population Approach Group in Europe (PAGE); 13–15 Jun 2007; Copenhagen.
Zhu Y, Hu C, Lu M, Liao S, Marini JC, Yohrling J, et al. Population pharmacokinetic modeling of ustekinumab, a human monoclonal antibody targeting IL-12/23p40, in patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2009;49(2):162–75.
Morton PA, Fu XT, Stewart JA, Giacoletto KS, White SL, Leysath CE, et al. Differential effects of CTLA-4 substitutions on the binding of human CD80 (B7–1) and CD86 (B7–2). J Immunol. 1996;156(3):1047–54.
Dustin ML, Starr T, Coombs D, Majeau GR, Meier W, Hochman PS, et al. Quantification and modeling of tripartite CD2-, CD58FC chimera (alefacept)-, and CD16-mediated cell adhesion. J Biol Chem. 2007;282(48):34748–57.
Kaymakcalan Z, Sakorafas P, Bose S, Scesney S, Xiong L, Hanzatian DK, et al. Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin Immunol. 2009;131(2):308–16.
Uchiyama S, Suzuki Y, Otake K, Yokoyama M, Ohta M, Aikawa S, et al. Development of novel humanized anti-CD20 antibodies based on affinity constant and epitope. Cancer Sci. 2010;101(1):201–9.
Carter P. Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer. 2001;1(2):118–29.
Chan AC, Carter P. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10(5):301–16.
International Nonproprietary Names (INN) for biological and biotechnological substances (a review). INN working document 05.179. Update 2011. http://www.who.int/medicines/services/inn/BioRev2011.pdf. Accessed 3 Dec 2012.
Acknowledgments
We thank James Kay for his assistance in the preparation of this manuscript.
Conflicts of interest
Iain Gardner, Rachel Rose and Manoranjenni Chetty are employees of Simcyp (now Certara). Miroslav Dostalek was employed at Simcyp (now Certara) at the time of preparation of this manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represents the view of F. Hoffmann-La Roche AG or the Centres for Disease Control and Prevention.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dostalek, M., Gardner, I., Gurbaxani, B.M. et al. Pharmacokinetics, Pharmacodynamics and Physiologically-Based Pharmacokinetic Modelling of Monoclonal Antibodies. Clin Pharmacokinet 52, 83–124 (2013). https://doi.org/10.1007/s40262-012-0027-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-012-0027-4