ABSTRACT
Purpose
To investigate the biodistribution of amphotericin B (AmB) in mice and rats following administration of liposomal AmB (AmBisome®) using a physiologically-based pharmacokinetic (PBPK) modeling framework and to utilize this approach for predicting AmBisome® pharmacokinetics in human tissues.
Methods
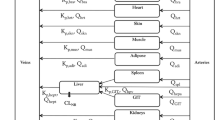
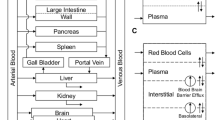
AmB plasma and tissue concentration-time data, following single and multiple intravenous administration of nonliposomal and liposomal AmB to mice and rats, were extracted from literature. The whole-body PBPK model was constructed and incorporated nonliposomal and liposomal subcompartments. Various structural models for individual organs were evaluated. Allometric relationships were incorporated into the model to scale parameters based on species body weight.
Results
A non-Michaelis-Menten mechanism was included into the structure of the liver and spleen liposomal compartments to describe saturable uptake of particles by the reticuloendothelial system. The model successfully described plasma and tissue pharmacokinetics of AmB after administration of AmBisome® to rats and mice.
Conclusions
The dual PBPK model demonstrated good predictive performance by reasonably simulating AmB exposure in human tissues. This modeling framework can be potentially utilized for optimizing AmBisome® therapy in humans and for investigating pathophysiological factors controlling AmB pharmacokinetics and pharmacodynamics.







Similar content being viewed by others
REFERENCES
Thornton SJ, Wasan KM. The reformulation of amphotericin B for oral administration to treat systemic fungal infections and visceral leishmaniasis. Expert Opin Drug Deliv. 2009;6(3):271–84.
Thornton SJ, Wasan KM, Piecuch A, Lynd LL, Wasan EK. Barriers to treatment for visceral leishmaniasis in hyperendemic areas: India, Bangladesh, Nepal, Brazil and Sudan. Drug Dev Ind Pharm. 2010;36(11):1312–9.
Adler-Moore J, Proffitt RT. Am Bisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J Antimicrob Chemother. 2002;49 Suppl 1:21–30.
Bern C, Adler-Moore J, Berenguer J, Boelaert M, den Boer M, Davidson RN, et al. Liposomal amphotericin B for the treatment of visceral leishmaniasis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2006;43(7):917–24.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
Gershkovich P, Wasan EK, Lin M, Sivak O, Leon CG, Clement JG, et al. Pharmacokinetics and biodistribution of amphotericin B in rats following oral administration in a novel lipid-based formulation. J Antimicrob Chemother. 2009;64(1):101–8.
Davidson EM, Barenholz Y, Cohen R, Haroutiunian S, Kagan L, Ginosar Y. High-dose bupivacaine remotely loaded into multivesicular liposomes demonstrates slow drug release without systemic toxic plasma concentrations after subcutaneous administration in humans. Anesth Analg. 2010;110(4):1018–23.
Adler-Moore JP, Proffitt RT. Amphotericin B lipid preparations: what are the differences? Clinical Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2008;14 Suppl 4:25–36.
Landsiedel R, Fabian E, Ma-Hock L, Wohlleben W, Wiench K, Oesch F, et al. Toxico-/biokinetics of nanomaterials. Archives of toxicology. 2012.
Li SD, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5(4):496–504.
Martinez MN. Factors influencing the use and interpretation of animal models in the development of parenteral drug delivery systems. AAPS J. 2011;13(4):632–49.
Harashima H, Komatsu S, Kojima S, Yanagi C, Morioka Y, Naito M, et al. Species difference in the disposition of liposomes among mice, rats, and rabbits: allometric relationship and species dependent hepatic uptake mechanism. Pharm Res. 1996;13(7):1049–54.
Gershkovich P, Wasan EK, Sivak O, Li R, Zhu X, Werbovetz KA, et al. Visceral leishmaniasis affects liver and spleen concentrations of amphotericin B following administration to mice. J Antimicrob Chemother. 2009.
Benson JM, Nahata MC. Pharmacokinetics of amphotericin B in children. Antimicrob Agents Chemother. 1989;33(11):1989–93.
Graybill JR. Is there a correlation between serum antifungal drug concentration and clinical outcome? J Infect. 1994;28 Suppl 1:17–24.
Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73.
Kagan L, Gershkovich P, Wasan KM, Mager DE. Physiologically based pharmacokinetic model of amphotericin B disposition in rats following administration of deoxycholate formulation (Fungizone®): pooled analysis of published data. AAPS J. 2011;13(2):255–64.
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13(4):407–84.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5.
Gerlowski LE, Jain RK. Physiologically based pharmacokinetic modeling: principles and applications. J Pharm Sci. 1983;72(10):1103–27.
Tsuji A, Yoshikawa T, Nishide K, Minami H, Kimura M, Nakashima E, et al. Physiologically based pharmacokinetic model for beta-lactam antibiotics I: tissue distribution and elimination in rats. J Pharm Sci. 1983;72(11):1239–52.
Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York: Marcel Dekker, Inc.; 1982.
Robbie G, Chiou WL. Elucidation of human amphotericin B pharmacokinetics: identification of a new potential factor affecting interspecies pharmacokinetic scaling. Pharm Res. 1998;15(10):1630–6.
Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob Agents Chemother. 2002;46(3):834–40.
Wang LH, Fielding RM, Smith PC, Guo LS. Comparative tissue distribution and elimination of amphotericin B colloidal dispersion (Amphocil) and Fungizone after repeated dosing in rats. Pharm Res. 1995;12(2):275–83.
Chow HH, Wu Y, Mayersohn M. Pharmacokinetics of amphotericin B in rats as a function of dose following constant-rate intravenous infusion. Biopharm Drug Dispos. 1995;16(6):461–73.
Matsui S, Imai S, Yabuki M, Komuro S. Pharmacokinetics characterization of liposomal amphotericin B: investigation of clearance process and drug interaction potential. Arzneimittelforschung. 2009;59(9):461–70.
Kawai R, Lemaire M, Steimer JL, Bruelisauer A, Niederberger W, Rowland M. Physiologically based pharmacokinetic study on a cyclosporin derivative, SDZ IMM 125. J Pharmacokinet Biopharm. 1994;22(5):327–65.
Harashima H, Kume Y, Yamane C, Kiwada H. Non-michaelis-menten type hepatic-uptake of liposomes in the rat. J Pharm Pharmacol. 1992;44(9):707–12.
Kume Y, Maeda F, Harashima H, Kiwada H. Saturable, non-Michaelis-Menten uptake of liposomes by the reticuloendothelial system. J Pharm Pharmacol. 1991;43(3):162–6.
Tollemar J, Ringden O, Tyden G. Liposomal amphotericin-B (Ambisome) treatment in solid organ and bone-marrow transplant recipients—efficacy and safety evaluation. Clin Transplant. 1990;4(3):167–75.
Ringden O, Meunier F, Tollemar J, Ricci P, Tura S, Kuse E, et al. Efficacy of amphotericin B encapsulated in liposomes (AmBisome) in the treatment of invasive fungal infections in immunocompromised patients. J Antimicrob Chemother. 1991;28(Suppl B):73–82.
Hong Y, Ramzan I, McLachlan AJ. Disposition of amphotericin B in the isolated perfused rat liver. J Pharm Pharmacol. 2004;56(1):35–41.
Angra PK, Siddig A, Nettey H, Desai N, Oettinger C, D'Souza MJ. Pharmacokinetic and biodistribution studies of amphotericin B microspheres. J Microencapsul. 2009;26(7):627–34.
Fielding RM, Smith PC, Wang LH, Porter J, Guo LS. Comparative pharmacokinetics of amphotericin B after administration of a novel colloidal delivery system, ABCD, and a conventional formulation to rats. Antimicrob Agents Chemother. 1991;35(6):1208–13.
Chow DD, Essien HE, Padki MM, Hwang KJ. Targeting small unilamellar liposomes to hepatic parenchymal cells by dose effect. J Pharmacol Exp Ther. 1989;248(2):506–13.
Li M, Al-Jamal KT, Kostarelos K, Reineke J. Physiologically based pharmacokinetic modeling of nanoparticles. ACS nano. 2010;4(11):6303–17.
Harashima H, Iida S, Urakami Y, Tsuchihashi M, Kiwada H. Optimization of antitumor effect of liposomally encapsulated doxorubicin based on simulations by pharmacokinetic/pharmacodynamic modeling. J control Rel Off J Control Rel Soc. 1999;61(1–2):93–106.
Liu D, Hu Q, Song YK. Liposome clearance from blood: different animal species have different mechanisms. Biochim Biophys Acta. 1995;1240(2):277–84.
Caron WP, Clewell H, Dedrick R, Ramanathan RK, Davis WL, Yu N, et al. Allometric scaling of pegylated liposomal anticancer drugs. J Pharmacokinet Pharmacodyn. 2011;38(5):653–69.
van Etten EW, Otte-Lambillion M, van Vianen W, ten Kate MT, Bakker-Woudenberg AJ. Biodistribution of liposomal amphotericin B (AmBisome) and amphotericin B-desoxycholate (Fungizone) in uninfected immunocompetent mice and leucopenic mice infected with Candida albicans. J Antimicrob Chemother. 1995;35(4):509–19.
Sivak O, Gershkovich P, Lin M, Wasan EK, Zhao J, Owen D, et al. Tropically stable novel oral lipid formulation of amphotericin B (iCo-010): biodistribution and toxicity in a mouse model. Lipids Health Dis. 2011;10:135.
Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob Agents Chemother. 2002;46(3):828–33.
Proffitt RT, Satorius A, Chiang SM, Sullivan L, Adler-Moore JP. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J Antimicrob Chemother. 1991;28(Suppl B):49–61.
Smith PJ, Olson JA, Constable D, Schwartz J, Proffitt RT, Adler-Moore JP. Effects of dosing regimen on accumulation, retention and prophylactic efficacy of liposomal amphotericin B. J Antimicrob Chemother. 2007;59(5):941–51.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 844 kb)
Rights and permissions
About this article
Cite this article
Kagan, L., Gershkovich, P., Wasan, K.M. et al. Dual Physiologically Based Pharmacokinetic Model of Liposomal and Nonliposomal Amphotericin B Disposition. Pharm Res 31, 35–45 (2014). https://doi.org/10.1007/s11095-013-1127-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1127-z