Abstract
Water purification is considered one of the most essential issues in our lives. Therefore, the contamination of water surfaces owing to the hasty upsurge in industrialization has received great global attention because of its potential danger to human health and the eco-system. Particularly, the discharge of various non-biodegradable contaminants into the water body—heavy metal ions, organic dyes, pharmaceutical antibiotics, pesticides, and oils—causes these contaminants to accumulate on the water's surface and have harmful impacts on humans and the environment. Several conventional methods can be applied to solve this problem, including chemical oxidation, precipitation, coagulation, and so on. However, they suffer from serious limitations: high cost, limited functionality, prolonged and heavy energy use, and poor separation efficiency. Conversely, the adsorption technique has recently attracted a lot of attention for wastewater treatment thanks to its remarkable benefits of being a simple, highly selective, and low-cost technique. Recently, natural polysaccharides (especially starch)-based adsorbents have received great interest in water purification owing to their outstanding properties, including being easily available, non-toxic, low-cost, biodegradable, and biocompatible. However, it possesses notable drawbacks that prevent it from being used alone as an adsorbent for wastewater treatment, including low thermal stability, slight water solubility, and rapid degradability in water. Therefore, this review highlights a comprehensive presentation about various starch modifications: starch-based grafts, hydrogels, aerogels, beads, nanofibers, and nanocomposite formulations to remove several toxic contaminants, including toxic heavy metal ions, organic dyes, pharmaceutical antibiotics, pesticides, and oils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water contamination is the most serious ecological concern because of the paucity of drinking water. Water pollution has fatal impact on human health and the health of other living organisms. Water purification is considered as a crucial topic in recent years. Rapid industrialization and significant growing population results in flowing of very dangerous industrial effluents and domestic sewage into water sources. They include many toxic materials that deteriorating and poisoning the water surfaces [1,2,3,4]. In this issue, there are different pollutants present in industrial effluents such as toxic heavy metals, synthetic dyes, residual antibiotic and pesticides, and different oil pollutants which are non-biodegradable materials and not degrade easily via conventional treatment techniques. As a result, they accumulate in living cells and cause fatal diseases [5,6,7].
To solve this environmental problem, wastewater can be purified by several conventional techniques such as chemical precipitation [8, 9], electrochemical technologies [10], ion floatation, [11, 12], membrane filtration [13], coagulation / flocculation [14,15,16], and reverse osmosis [13, 17, 18], and microbiological techniques [19]. However, conventional techniques have significant drawbacks such as limited function, long time and high energy consumption, high cost and low separation efficiency. Recently, the adsorption method has gained intensive interest for wastewater treatment owing to its outstanding advantages such as easy operation, low-cost, and high selectivity [5, 20, 21].
To date, many scientific research groups worldwide are interested in preparing efficient adsorbent based on natural biopolymer polysaccharides for wastewater treatment to overcome the current challenges. Polysaccharides are natural carbohydrates which consist of different monosaccharide units (glycans), and/or sugar derivatives (heteroglycan) bonded together via glycosidic bonds [22]. They have remarkable features such as easily available and have excellent properties such as non-toxic, biodegradable, biocompatible, and amenable to modifications [23,24,25]. Therefore, they are harmless to human and eco-system and hence, they are widely applied for different essential fields such as wastewater treatment, food, textile, cosmetic, paper, and medicine fields [22, 26,27,28,29]. Particularly, starch-based adsorbent has been attracted a lot of attention in the field of wastewater treatment from different toxic pollutant due to its excellent properties, including non-toxicity, biodegradability, and low-cost material [30]. However, unmodified starch is rarely applied directly as adsorbent for wastewater treatment because it has certain notable drawbacks, including low surface area, poor water solubility, low molecular weight, limited thermal stability, and lack of reaction function groups. Therefore, several starch modifications have been fabricated to improve its adsorption efficiency toward different pollutants via adding functional groups on starch backbone and changing its chemical surface [20].
Many recent reviews have demonstrated explored the application of modified starch for wastewater treatment. Fang et al. [20], have studied the removal of dye, heavy metals, pharmaceutics, and oil by using starch-based magnetic adsorbents. While Haq et al. [31], have presented the use of starch-based hydrogels, composites and nanoparticles for removal of dyes and heavy metals. Moreover, Gupta et al. [32], have focused on the starch modification with different techniques: oxidation, irradiation, grafting, crosslinking and esterification techniques for removal of heavy metals. On the other hand, this review article highlights a comprehensive presentation about the recent advances in the fabrication of starch modifications such as starch-based grafted copolymers, hydrogels, polymer nanocomposite, aerogels, beads, and nanofibers, to remove various toxic pollutants: toxic heavy metal ions, synthetic dyes, pharmaceutical antibiotics, pesticides, and oils. It covers several starch formulations for removal of various toxic pollutants via adsorption technique.
Starch
Starch Structure
Starch, low-cost second abundant polysaccharide, is a homopolymer of Glucopyranose units with the molecular formula (C6H10O5)n. Amylose and amylopectin are two different forms of polymer chains that make up starch as shown in Fig. 1. [33]. While amylopectin is a branching polymer consisting of -1,4-glycosodic with branched chain linked by -1,6-glycosidic bonds, amylose is a straight chain -1,4-glycosidic links. Each of these polymers receives unique features as a result of this conformational change. For instance, the crystalline area of the granules is caused by the short branching of amylopectin at the-1,6-glycosidic linkages [34, 35]. About 20–30% of starch is amylose and 70–80% is amylopectin in its natural state. Starch is mainly synthesized in the chloroplast of plant leaves and/ or in the amyloplast of the storage organs of plants. It contains some lipids and phosphate groups. There are various reported botanical sources of starch such as corn starch, rice starch, potato starch and Cassava Starch [36, 37].
Chemical illustration of starch structure [38].
Physicochemical Properties of Starch
Indeed, starch has many wonderful benefits, like biocompatibility, safe usage, low-cost, biodegradability, and ease of availability. Moreover, it consists of several hydroxyl groups (Fig. 1). Starch has relatively poor water solubility and a relatively lower capacity to absorb water and oil due to its polymeric and branching structure. Additionally, it has an excellent swelling power, good gelatinization powers, and a relatively high viscosity. It may also create thin films and has good pasting qualities, including consistency, smoothness, and clarity. Starch is increasingly being used to remove water and environmental pollutants such colours, heavy metals, pesticides, antibiotics, and microbes [39]. The functional qualities of starch can be modified under the influence of numerous physical and chemical conditions to boost its nutritional, biomedical, and environmental relevance. However, it has some drawbacks that make it inappropriate to be utilised as an adsorbent for wastewater treatment without modification, including limited thermal stability, being damaged by several of microbes, and rapid degradability in water. The modification of starch to create highly effective adsorbents for wastewater treatment applications has thus been the subject of investigation by a number of scientists.
Chemical Modification of Starch
Due to the presence of several hydroxyl groups on the surface of starch, it is an ionic hydrophilic linear polymer with fabulous features such as non-toxicity, biodegradability, biocompatibility, and low-cost material. Nevertheless, native starch has certain notable drawbacks, including low surface area, poor water solubility, low molecular weight, limited thermal stability, and lack of reaction function groups, which represent obstacles to apply it directly as adsorbent for wastewater treatment [20]. In this review, several starch modifications have been fabricated such as starch-based grafts, hydrogels, polymer nanocomposites, beads, aerogels, and nanofibers to overcome its limitations to enhance its adsorption ability for wastewater treatment.
Grafted Starch
Grafting is the process of covalently bonding monomers to polymeric chains, which is followed by further polymerization of those chains to create the desired product [40, 41]. Increasing the nonpolar nature of starch is a frequent technique [42, 43]. Grafted co-polymers and starch biopolymers have a wide range of uses, including the treatment of waste water and the delivery of drugs and genes for tissue engineering and gene therapy [32, 44]. Three more techniques can be used to produce starch-grafted synthetic polymers: grafting through, grafting to, and grafting from [42, 43, 45, 46]. Copolymerization of macromonomers occurs during the grafting process. Grafting onto involves a reaction between two distinct functional groups on independent polymeric strands. In the grafting-onto approach, the interaction between the functional groups on the polymeric chains causes the polymerization of vinyl monomers to begin. [38, 41]. The grafting from method is the most popular of these three methods because it gives access to the polymeric chains' reactive groups and produces a high yield of grafted starch (Fig. 2) [38, 41, 42]. In the grafting through (macromonomer) method the macromonomers are oligomeric or polymeric chains bearing a polymerizable end group. Copolymerization of preformed macromonomers with another monomer yields graft copolymers. Table 1 shows the advantage and disadvantage of different techniques of the grafted methods [47, 48].
Different routes in graft copolymerization [48].
For example, varied irradiation doses and dosage rates were employed to create copolymers of starch-graft-polyacrylamide (St-g-PAM) using electron beam irradiation as the free radical initiator. Using an accelerated electron beam, St-g-PAM filled with freshly manufactured silver nanoparticles was also created. The results revealed that the level of grafting substantial viscosity and thermal behaviour (thermodynamic parameters) were influenced by the irradiation dose, dose rate and presence of silver nanoparticles. The coagulation–flocculation process of the synthesized copolymers in aqueous solutions showed good efficiency to improve different water quality indicators [49]. In order to meet the need for treating coal mine wastewater, acrylamide and legume starch were combined to create a novel, very effective flocculant. According to experimental findings, the classic polyacrylamide and polyaluminum chloride does not possess the same excellent flocculation effect as the graft copolymer of acrylamide and mung bean starch. The new flocculant has a number of advantages, including being inexpensive, quick to degrade, and especially appropriate for situations with strict environmental standards. It is also likely to have a wide range of potential applications for treating coal mine wastewater [44]. For example, in order to remove Pb2+ ions from aqueous media using a batch approach, Ekebafe et al. [50] described the synthesis of hydrolyzed cassava starch-g- poly (acrylic acid) (St-g-PAA) and starch-g-poly (acrylonitrile) (St-g-PAN) copolymers that adsorbed Pb2+ ions in aqueous solution via chelation mechanism. The prepared adsorbents showed maximum adsorption capacity of Pb2+ ions reached 118.61 mg/g for St-g-PAA and 115.83 mg/g for St-g-PAN at optimized conditions, contact time (18.25 h), temperature (300.3 K), pH (7.85), 20 ppm of Pb2+ concentration, and an adsorbent dose of 0.5 g/100 ml. The adsorption experimental data fitted very well with the Elovich equation and the intra-particle kinetic models.
Starch-Based Hydrogels & Beads
Hydrogels are a type of three-dimensional polymeric structure which has a significant water imbibing capacity. Hydrogels exhibit a large scale of flexibility in terms of fast swelling capacity, modifiable surface properties such as charge, functionality, fast dispersion process, large surface area, porous structure, permeability, catalysis, fast kinetics, thermal stability, wonderful acid/base properties and hydrophilicity [51]. When compared to other adsorbents, hydrogels are preferred in adsorption applications due to their quicker kinetics and greater capacity to remove a variety of pollutants [47]. Starch's structure includes several hydroxyl groups that help hydrogel networking. Starch-based hydrogels (SHs) have drawn a lot of attention since they are widely available and have numerous applications [52].
Because hydrogels made of synthetic polymers and petrochemicals are not environmentally friendly, polysaccharides like starch are used to create biodegradable hydrogels. By cross-linking, hydrolyzing, and polymerizing the material's vinyl monomers that have been aggregated with one or more functional groups, hydrogels are created. Different components can be included within the polymeric matrix to lower costs and increase adsorption effectiveness [47]. The presence of hydrophilic groups as –COOH, –SO3H, –OH, and –NH2 increases the hydrophilicity of hydrogel polymer. Starch hydrogels (SHs) gained huge attention in many fields due to the presence of a large number of hydroxyl groups. SHs also are non-toxic, biocompatible, and cheap. Due to these reasons, SHs can be an alternative to synthetic [52]. Starch hydrogels for wastewater treatment are modified by incorporation of functional groups such as primary amine, hydroxyl, carboxylic or sulfonic acid that enhancing its pollutants removal capacity [53].
For instance, modification by graft copolymerization is widely studied and used synthetic methods in HGs composed of polysaccharides [54]. Recently, Farag and co-workers synthesized bifunctional HG using radiation-induced grafting method. 2-Acrylamido propanesulphonic acid and dimethylaminoethyl methacrylate was copolymerised grafted onto starch and modified using benzyl chloride. These gels exhibited excellent removal efficiency for dye (astrazon red), cobalt, and phosphate with maximum adsorption capacities (Qmax) of 600 mg/g, 426 350 mg/g, and 650 mg/g, respectively [55]. In another work, to improve the water capacity of starch hydrogel, Kirti et al. prepared a starch hydrogel with enhanced water-absorbing capabilities by glutaraldehyde crosslinking of starch with PVA and blending with 1-Butyl-3-methylimidazolium tetrafluoroborate (BMIM-BF4) to enhance the plasticity and generate porosity within the hydrogel, leading to swelling capacity up to 300% [56]. Whereas, a poly(m-aminothiophenol) (MAP)/potato starch hydrogel for the elimination of Hg2+ ions from wastewater was reported by Fu et al. [57]. Following inverse vulcanization, sulfur interacts with MAP/potato starch to create crosslinked cp MAP-S-PS hydrogel for removal of Hg2+ (Fig. 3a), the adsorption mechanism of cp MAP-S-PS could be attributed to the formation of a coordinated band or complex between Hg2+ ions and various donor atoms (S, N, and O). The obtained hydrogel displayed excellent selectivity for Hg2+adsorption with Qmax of 436.68 mg/g at conditions of pH (4.5), temperature (328 K), and contact time (180 min). While the regeneration study showed that the adsorption efficiency decreased to 197.77 mg/g after fifth cycle as shown in Fig. 3b.
a Chemical illustration of crosslinked MAP-S-PS hydrogel for removal of Hg2+ ions, and b the adsorption capacity of crosslinked MAP-S-PS hydrogel through five reusable cycles [57].
On the other hand, starch beads are 3D crosslinked hydrogel in spherical shape structure. They have been favoured for industrial fields owing to its uniform size, low friction, and low pressure drop in the columns [58]. The uses for micro- and nanoscale beads and spheres are numerous, but they are particularly useful in the environmental and medical fields. As an illustration, nanobeads and spheres frequently have a high mechanical stability, a low weight, and a large specific surface area [59]. Most recently, amidoximated starch hydrogel beads were prepared by Diana et al. [60] with ionotropic gelation method in the presence of cationic chitosan (Fig. 4a and b). Batch experiments were used to test the hydrogel beads' capacity for solubilizing heavy metals such as Ni2+, and Zn2+ and two organic dyes including direct blue 15 and congo red in simulated polluted water in relation to the characteristics of the beads. Figure 4c showed optical image of the prepared beads, and Fig. 4d exhibited spherical shape for modified starch beads.
The chemical structure of a amidoximated starch and b chitosan, and c optical image of the prepared hydrogel beads (scale bar 5 mm) and d SEM images of modified starch hydrogel beads (scale bar 20 µm) [60].
Starch-Based Polymer Nanocomposites
It has been noted that polymer nanocomposites with inorganic reinforced fillers of nanoscale size are efficient reinforcing agents that considerably enhance the physical and chemical properties of the matrix [61]. Because of the reduction in the total free energy of mixing, nanofillers are known to have good compatibilization and miscibility with various polymer matrixes and exhibit high performance at low concentration. The dispersion and localization of nanofillers, which may be evenly distributed throughout the polymer matrix or concentrated at the interface, affect the characteristics of polymer nanocomposites. Several researches have been done on starch-based polymer nanocomposite materials that are filled with various nanofillers, carbon nanomaterials (such as graphene and its derivatives, and CNTs), metal/metal oxides, and silicate nanoclays [62]. The findings demonstrate that nanofillers enhance mechanical and thermal stability due to their superior dispersion inside the matrix polymer [62].According to earlier studies [43, 44]. For example, Alperet al. [63], exfoliated graphene oxide (GO) nanosheets within hydroxyethyl starch (HES)/divinylsulfone nanocomposite to remove malachite green dye. The obtained findings showed maximum adsorption of the prepared nanocomposite of 89.3 mg/g. In another study, starch nanocomposites were created by Abdul-Raheimet al. [64], from modified starch, acrylic acid, and Fe3O4for removal toxic heavy metal ions such as Pb2+, Cu2+, and Ni2+ ions. The findings showed that maximum adsorption reported as 100, 70 and 100 mg/g for Pb2+, Cu2+, and Ni2+ ions, respectively. Moreover, Mohammed et al. [65], reported the preparation of Fe2O3 NPs /starch nanocomposite using formaldehyde as a crosslinker (Fig. 5) for removal different toxic divalent cations: Pb2+, Hg2+, and Cd2+ ions. The adsorption of metal ions was accomplished by starch-based iron oxide nanoparticles with many OH groups and the schematic diagram to represent the synthetic procedure of Fe2O3 NPs-starch nanocomposite and the proposed core shell structure of Fe2O3 NPs-starch nanocomposite. The results showed Qmax of Hg2+, Pb2+, and Cd2+of 133.3 mg/g, 2000 mg/g, and 322.58 mg/g, respectively, at the optimal conditions for maximal adsorption: pH 7, 20 min of contact time, 0.2 mg/l of metal, and 20 mg of adsorbent. The findings showed that Pb2+ exclusively obeyed the Freundlich isotherm model, while Hg2+ and Cd2+ were best fitted by all models.
Schematic preparation of modified starch/ Fe2O3 NPs polymer nanocomposites [65].
Starch-Based Aerogels
Aerogel formulations are 3D porous materials and are considered one of the most promising adsorbed materials for water purification from various pollutants such as heavy metal ions, dyes, etc. [66]. They are classified into three categories: organic, inorganic, and hybrid forms whose porous micro- or nanostructure [67, 68]. The physico-chemical characteristics of the prepared aerogels are primarily controlled by several parameters as starch supply, biopolymer content, amylose/amylopectin ratio, and degree of gelatinization [69]. They have outstanding features: low-cost, sustainable, and potential materials as well as high surface area, high porosity, and low density [70,71,72]. Starch-based aerogels are a class of advanced porous materials which are easily fabricated with different biopolymers, and/or synthetic polymers via freeze drying technique [73]. For example, recently, Li et al. [74], have fabricated rich 3D layered porous starch-based aerogel (starch/polyacrylamide/CaCO3/ TEMPO-oxidized nanocellulose) via freeze drying method for removal of MB and CR dyes from an aqueous solution (Fig. 6). The resultant starch aerogels had excellent swelling ability as well as potential adsorption capacity for MB and CR reached 101.01 mg/g and 277.76 mg/g, respectively, via electrostatic and H-bonding interactions. After four reusable cycles, the adsorption efficiency of the starch aerogels decreased to 74.56% and 65.75% for CR and MB, respectively.
Schematic preparation of 3D layered porous porous starch-based aerogel (starch/polyacrylamide/CaCO3/ TEMPO-oxidized nanocellulose) via freeze drying method for removal of MB and CR dyes from an aqueous solution [74].
In another study, Yun et al. [75] have prepared highly porous aerogels via combination of reduced graphene oxide (rGO) nanosheets with dialdehyde starch nanocrystals (DASNC) via freeze drying method (Fig. 7). After modification of DASNC with rGO, the findings found that the prepared aerogel surface with high robust, which resulted in superior mechanical property, as well as excellent specific capacitance reached up to 316 F/g. Moreover, the obtained aerogels were applied as adsorbent for removal of rhodamine B (RB) and crystal violet (CV) with high Qmax reached up to 539 mg/g and 318 mg/g for RB and CV, respectively, owing to the porous surface and high surface area of the examined starch aerogels.
Fabrication of modified starch/rGO aerogels structure; the chemical structure of a DASNC, b GO, and c DASNC/ rGO aerogels [75].
Starch-Based Nanofibers
Polymeric nanofiber membranes can be designed to widely apply for removing various contaminants from polluted water owing to its significant features such as large surface area, high separation efficiency, high porosity, low-cost and easily functionalize with multifunctional materials to achieve potential wastewater treatment [76].The fabrication methods of polymeric membranes include electrospinning, phase inversion, track -etching, sintering and stretching method [77]. However, electrospun nanofibers have received potential attention in wastewater treatment compared with other approaches because of its advanced advantages including simple modification technique, strong versatility, low-cost method, and broad selection of materials. In this approach, charged polymer melt of solution and high-voltage electric field are applied to design smooth and controllable morphology nanofibers [78, 79]. Recently, for the removal of Cr6+ions from an aqueous solution, Sun et al. [80] have prepared electrospun Starch-graft-Polyacrylonitrile (St-g-PAN) nanofibers via electrospinning technique in in dimethyl sulfoxide (Fig. 8). In this study, the authors charged about 4.3 wt% of grafted starch under typical spinning voltage of 15 kV. The SEM image displays finer fibrous without any knobs. Moreover, the adsorption results demonstrated that the obtained St-g-PAN nanofiber achieved potential removal of Cr6+ ions with Qmax of 533.4 mg/g, at pH 2.0. Table 2 showed the advantages and disadvantages of different mentioned starch modifications.
Schematic preparation of electrospun Starch-graft-Polyacrylonitrile (St-g-PAN) nanofibers via electrospinning technique in in dimethyl sulfoxide [80].
Modified Starch-Based Adsorbents for Wastewater Treatment
Excessive leakage of various contaminants into the water surfaces owing to rapid urbanization and industrialization as well has received a great interest worldwide. Among these contaminants, heavy metal ions, textile organic dyes, residual pharmaceutical antibiotics, pesticides, and oils, which are highly toxic, and non-biodegradable materials. As a result, they significantly reduce water quality, accumulate in living cells, and cause fatal diseases. Therefore, in recent years, wastewater purification has been received great attention from worldwide scholar researchers. Recent modified starch-based adsorbents possess many advantages including high adsorption selectivity, good thermal and mechanical stability, and low-cost materials, which increase its capability for water purification [88].
Removal of Heavy Metal Ions
Heavy metal ions (HMs) define as elements have an nuclear density more than 4 ± 1 g/cm3 [13, 17]. HMs such as, cadmium (Cd), chromium (Cr), iron (Fe), lead (Pb), cobalt (Co) and nickel (Ni) ions are commonly used in industrial applications whose industrial waste products are drained into water streams. However, they have many obstacles, like high toxicity, carcinogenicity, water-solubility, and non-biodegradability. Consequently, their effluents contaminate all water resources, and then accumulate in living cells and result in a dangerous influence on human health. They can also lead to Parkinson's and Alzheimer's illnesses and neurodegenerative disorders [17, 89,90,91,92,93].
Heavy metals enter the water sources from a diversity of industries, such as minerals extraction, water treatment, metal molding, metal coating, batteries, nuclear industry, Leather tanning, electroplating, fuel combustion, landfills, electronics, metal finishing, wood processing, enamelling and nuclear power generation [17, 18, 94,95,96,97]. Adsorption technique isa considered as the most effective method for eliminating various HMs from contaminated water owing to its low-cost, ecological impact, excellent removal efficiency and recyclability, and reliability [98, 99]. Modified starch is an excellent metal ion adsorbent due to its large number of hydroxyl functional groups. For instance, starch grafted copolymer-based adsorbents were applied for elimination different HMs from contaminated water. According to Mgombezi and Vegi [100] grafted potato starch (GPS) with poly(methyl methacrylate-co-oxalic acid) was prepared radical polymerization approach as an adsorbent for the removal of hardness ions (Ca2+ and Mg2+). The adsorption findings displayed Qmax of 74.50% for both metals at 80 min fitted with the Langmuir isothermal and a pseudo-second order kinetic model. In another study, for the elimination of Cu2+, Ni2+, Cd2+, and Zn2+ ions from water, Ibrahim and Fakhre [101] reported the preparation of corn starch grafted with dibenzo-18-crown-6 under microwave irradiation. The findings showed that Qmax were observed to be 28.1, 24.5, 30.8, and 17.9 mg.g−1 for Zn2+, Cd2+, Ni2+,and Cu2+ ions at pH6.5, respectively, via electrostatic interaction.
According to literature, addition of functional groups into starch polymeric chains modified by grafting exhibited good adsorption features towards HMs. For instance, Chen et al. [102] mentioned polyamine-type corn starch/GMA grafted copolymer with a high capacity for Cu2+, Pb2+, Cd2+, and Cr3+ ions. According to the reported results, Qmax were observed to be 2.33, 1.25, 0.83 and 0.56 mmol.g−1 for Cu2+, Pb2+, Cd2+, and Cr3+, respectively, at pH 5.0, and 12 h via coordination bonds between the adjacent amine groups in polymer chains and metal ions.in addition. The regeneration study showed excellent reusability, as the adsorption capacity remained stable after 10 reusable cycles.
While Aniagor and Hashem [103], described the preparation of polyacrylonitrile (PAN) grafted corn starch to remove Zn2+ ions. Grafted PAN including polar CN groups provide entrenched stable network chains and enhanced both of water absorption capacity and cation binding interaction. The high adsorption capacity of Zn2+ ions 508.85 mg/g was demonstrated at pH of 5.0, 20 min, and 0.3 g/l adsorbent concentrations.
In addition to grafted starch copolymers, the starch-based hydrogels including great attention to be an efficient adsorbent for elimination of toxic HMs from contaminated water. Farag et al. [55] used radiation method to graft copolymerize 2-acrylamido-2-methylpropane-1-sulphonic acid (AMPS) and dimethyl aminoethyl methacrylate (DMAEMA), for capturing of Co2+ ions. The findings illustrated that Qmax was observed of 350 mg/g at 25 °C, and pH 3. the Co2+ ions adsorption of onto the obtained hydrogels is via coordination bond between NH2 groups on hydrogel surface and lone pair of electrons of Co2+ ions.
Moreover, Duquette and Dumont [104] demonstrated the preparation of grafted starch hydrogel in the presence of itaconic acid using acrylamide as a cross-linker. The obtained hydrogel had the ability to eliminate Cu2+ from the aqueous solution by Qmax of 1.36 mmol/g at pH 3.5. through electrostatic interaction between carboxylate anions and metal ions.
Whereas, according to Schmidt et al. [105], cross-linked potato starches hydrogels (StMBA) and starch-grafted PAM copolymer hydrogels were synthesized by chemical crosslinking polymerization (Fig. 9a) using MBA as a crosslinker for the removal Cu2+ and Fe3+ions from aqueous solutions. According to LSM image, alamellar morphology (Fig. 9b). The adsorption results demonstrated that StMBA had an adsorption efficiency of 14.0 mg/g (Fig. 9c) and 2.9 mg/g (Fig. 9d) for Cu2+ and Fe3+, respectively, while grafted starch hydrogels reported higher efficiency reached 23.0 mg/g and 21.2 mg/g for Cu2+ and Fe3+, respectively, via coordination bonds between metal ions and amine groups on the surface of starch hydrogels. In another study, Rahmanet al. [106] mentioned the preparation of a superabsorbent hydrogel based on potato starch and PAA via irradiation grafting approach to remove Cr6+ ions from industrial wastewater. The as-prepared hydrogel showed excellent adsorption capacity of 10.13 mg/g after 3 h. In order to remove heavy metal ions like Pb2+, Zn2+, and Cu2+, Anghelet al. [107] described the production of potato starch/cellulose / toluene-diisocyanate via batch-adsorption technology. The obtained data showed the Qmax of Pb2+, Zn2+, and Cu2+ were 66.66, 58.82, and 47.61 mg/g, respectively at pH 5.0, and 120 min, which well-fitted Langmuir isotherm model.
a Schematic representation of formation of cross-linked starch-g-PAM hydrogels, b SEM image of the prepared hydrogel, and the adsorption efficiency of c Cu2+ ions and d Fe3+ ions against contact time (min) [105].
Starch-based beads have been widely applied for removal of heavy metal ions from polluted water. For the elimination of Cu2+ ions, Dragan et al. [108] reported the preparation of grafted starch/polyamidoxime/ chitosan beads by ionotropic gelation. The adsorption data showed that Qmax estimated by the Langmuir model was 238.14 mg Cu2+/g for the fabricated beads at 25 °C. The adsorption mechanism exhibited that chemisorption is a mean mechanism of interaction between the chelating beads and the Cu2+ ions. Moreover, the prepared beads reused up to 5 adsorption–desorption cycles without any decrease in Cu2+ ions adsorption. While for the elimination of Pb2+ ions from an aqueous solution, Chen et al. [109] mentioned the preparation of starch/humic acid beads via inverse phase suspension crosslinking. The findings showed that Qmax was 50 mg/g at pH 5, and 298 k. The obtained beads showed good regeneration with little loss of adsorption capacity. The adsorption mechanism of Pb2+ ions was with electrostatic interaction. Recently, Dragan and Loghin [110] have fabricated starch beads based on starch-g-PAN/chitosan for the elimination Cu2+, Ni2+, and Co2+ ions from an aqueous solution. The adsorption findings showed that Qmax of Cu2+, Ni2+, and Co2+ ions onto the beads surface were 100.6 mg/g, 83.25 mg/g, and 74.01 mg/g, respectively via chemisorption mechanism (Fig. 10).
Schematic adsorption mechanism of heavy metals on the prepared starch beads adsorbent [110].
Starch polymer nanocomposites have great attention for purification the contaminated water from eluted industrial toxic heavy metal ions in recent years. The combination of starch and metals and /or metal oxides can provide new material with special mechanical and chemical properties as well as an improved metal ion adsorption capacity [111]. For instance, Saberiet al. [112], demonstrated the creation of starch/PEG-PAA hydrogel and AgNPs-base nanocomposite for removal of Hg2+ions. The Qmax was achieved as 158.21 mg/g and 182.53 mg/g in pH 7 and 6, respectively. A new nanocomposite mixed made of molybdenum trioxide (MoO3), waste mallow stem biochar, super magnetic spinel ferrite (MSB@CoFe2O4), and gelatin-esterified starch was designed and fabricated by Mahmoud et al. [113]. The results show the Qmax of Pb2+ onto hydrogels were 2075, 4375, and 5000 μmol.g−1, respectively, via electrostatic interaction mechanism. The reusability test showed a little loss (2%) after five continuous recycles.
In order to removal of Cu2+ and Pb2+ from wastewater, Saberiet al. [114] described the synthesis of the magnetic Fe3O4-based starch-PAA (Fe3O4@St-PAA) nanocomposite. The obtained Fe3O4@St-PAA showed the adsorption efficiency of 99.32% and 93.83% towards Cu2+, Pb2+, respectively, according to Langmuir isotherm model. The adsorption efficacy based on the electrostatic interaction between metal ions and various chelating groups in the magnetic nanocomposities such as carboxylic and hydroxyl groups.
In order to remove As3+ ions from aqueous solution, Bisht and Neupane [115], demonstrated the green chemical production of Fe3O4@St nanocomposite by supercritical CO2-assisted. The results showed that the maximum As3+ adsorption capacities on the obtained nanocomposite reached 124 mg/g via coordination covalent bond between As3+ ions and surface anions on magnetic surface. While the results showed that a complete removal of arsenic from the ground water sample in 15 min the nanomaterials be regenerated in NaOH solutions and could retain more than 50% of the As3+ removal capacity even in the fifth regeneration/reuse cycle. Moreover, Xie et al. [116] mentioned the preparation of Fe3O4/starch /PAA/PAM nanocomposite via an inverse emulsion graft copolymerization for elimination of Cd2+ ions from aqueous solution (Fig. 11). According to the findings, the maximum adsorption capacity of Cd2+ on the obtained nanocomposites was 39.98 mg/g, well described by the pseudo-second-order kinetic and Langmuir isothermal adsorption models. The obtained nanocomposite exhibited a various active sites on the surface as ‒COOH,‒OH, and‒NH functional groups that increase the chelation ability with Cd2+ ions.
Schematic diagram of Cd2+ adsorption by Fe3O4/starch /PAA/PAM nanocomposite [116].
Ahamad et al. [117] developed starch/salicylaldehyde/ Fe3O4 nanocomposite for the elimination of Pb2+ and Cd2+ from pollutant water. According to the adsorption data, the highest adsorption capacity for Pb2+ and Cd2+ ions was found to be 265.4 and 247.2 mg/g, respectively. After seven cycles, the manufactured starch nanocomposite samples showed an outstanding reusability.
While, according to Chen et al. [118], a new modified calcium and starch- ferromanganese binary oxide (Ca-SFMBO) nanocomposite adsorbent for the removal of arsenic As3+ and Cd2+ ions from water has been developed. The Qmax of % Ca-SFMBO for As3+ and Cd2+ of were 156.25 mg/g and 107.53 mg/g respectively at pH of 6. Moreover, Xu et al. [119] demonstrated the preparation of a new potato starch-FMBO with an Fe/Mn ratio of 1.00/2.00 and pH of 2 using starch for stabilization dispersion. The maximum As3+ adsorption capacity of starch-FMBO was 161.29 mg/g at 24 h.
The synthesis of a silica-sand/anionized corn starch composite for the removal of Cu2+ from water was reported by Li et al. [120]. The adsorption data showed that Qmax for Cu2+ ions of 383 mg/g at 20 min. The adsorption data are well fitted with Langmuir isotherm model the pseudo-second order kinetic.
Carbon-based nanomaterials are considered as the most attractive nanofillers which are used widely to incorporate inside polymer chains to enhance the adsorption efficiency of the used polymer [121]. In order to remove Cr6+, Kumar athilaka et al. [122] created a new starch-stabilized nano zerovalent iron-GO nanocomposite. GO was prepared by the Hummer method. The findings showed that maximum adsorption capacity of the obtained nanocomposite was 143.28 mg/g at pH 3, within 20 min, fitted well with the Langmuir isotherm model. In another study, Hosseinzadeh and Ramin [123] created Fe3O4/starch-g-PAM/GO nanocomposite via in situ radical solution polymerization to effectively remove Hg2+ from aqueous solution. The adsorption data found that nanocomposites had Qmax of 277 mg/g at optimum pH7. The Hg+2 removal mechanism was a result of the electrostatic interaction between the negatively charged carboxamide fictional groups and Hg+2 ions, as well as mercury ion covalently bonded to ‒NH groups of acrylamide. Moreover, the oxygen containing hydroxyl groups were complexed with Hg+2 ions. By using a simple esterification technique, Wang et al. [124] created starch/GO nanocomposite for elimination of Cd2+ from the aqueous solution. GO and poly PVA were esterified to produce the polymeric modification of graphene. The findings showed that maximum Cd2+ adsorption capacities were 43.20 mg/g well-fitted with the Langmuir isotherm model. Additionally, GO-starch nanocomposite was created by Bulin et al. [125] using a hydrothermal method to remove Cu2+ ions. The adsorption efficiency reached 542.1 mg/g at optimum pH 8. In the third cycle, the Qmax decreased to 372.19 mg/g. Also, in another study, starch/GO nanocomposite was fabricated by Guo et al. [126] to scavenge aqueous Pb2+ ions. The adsorption results showed that the obtained nanocomposite had a capacity of 383.32 mg/g (95.83%) of Pb2+ ions by Freundlich model. Moreover, the regeneration assay decreased the efficiency of the obtained nanocomposite to 189.69 mg/g in the fifth cycle. Moreover, by the same approach, A starch/GO nanocomposite membrane was designed by Beeviet al. [127] for removing Pb, Cd, Zn, and Mg ions from wastewater. The results showed that the maximum efficiency for heavy metal ions removal was 89.04%, 91.32%, 88.56%, and 89.76% for Pb, Cd, Zn, and Mg ions, respectively.
Starch nanocomposite-based on agricultural waste adsorbents are highly recommended in last few decades for water purification. Baghbadoran et al. [128] demonstrated the fabrication of cellulose nanofibers (CNFs) treated starch-g-PAA/MBA nanocomposites to remove Cu2+ ions from aqueous solution. The results showed that the maximal adsorption capacity of the fabricated nanocomposite was 957 mg/g, via ion exchange and chelating of Cu+2 ions and ionized&deionized carboxyl groups on nanocomposite surface (Fig. 12). Furthermore, lately, Mohamed et al. [129] have mentioned the preparation of Pisumsativum pods (N-PSPB)/ starch/glycerol nanocomposite for the removal of Cr6+ions. The surface area and hole volume of the obtained nanocomposites were 226.94 m2/g and 9.88 cm3/g, respectively, using SEM and BET techniques. The as-prepared nanocomposites achieved excellent adsorption efficiency up to 420.13 mg/g. They were able to maintain their adsorption efficiency even after 10 adsorption cycles.
Adsorption mechanism of Cu+2 ions on starch-g-PAA/MBA/CNFs nanocomposites [128].
On the other hand, silicate nanoclays are widely applied for removing toxic heavy metals from contaminated water. Silicate nanoclay which resulting from isomorphous substitution, and have the capacity to adsorb HMs via exchange of their counter cation [130]. For example, according to Chen et al. [131], Laponite RD/corn starch/PVA nanocomposite was synthesized by freezing/thawing method for the removal of Cd2+ ions from wastewater. The findings showed excellent cadmium ion adsorption efficiency of 63.46% was obtained when LRD was at 12.5% after 24 h. Moreover, the adsorption of Cd+2 ions was attributed to the electrostatic interaction between the negative charged surface of nanoclay and Cd+2 ions. The intercalation process was reported by Garca-Padilla et al. [132], to synthesize starch/MMT nanocomposites with varied starch-to-nanoclay ratios: 5:1, 10:1, and 10:3 in acetic acid solution to remove Ni2+ and Co2+ ions from aqueous solution. The results showed the highest removal of Ni2+ and Co2+ ions were 97.1%, and 78.7%, respectively, according to Langmuir model. Most recently, starch-based aerogels have been applied for removal of heavy metals from polluted water. For example, Camani H. and his team-work [133] have reported crosslinked corn starch adsorbent aerogels in the presence of citric acid as a crosslinking agent via freeze-drying method for removal of Cd2 + and Zn2 + ions from an aqueous solution. The adsorption data showed good adsorption efficiency for the obtained starch aerogels owing to high porosity and pore density, large specific area, and good mechanical stability. The results reported that adsorption efficiency reached 30% at 12 h via ion-exchange mechanism (Fig. 13 a and b). Several reported studies in recent years have been mentioned based on different starch formulations for removal of different toxic heavy metals (Table 3).
Schematic of the adsorption of a Cd2+ ions b Zn2+ ions on crosslinked corn starch aerogels [133].
Removal of Synthetic Dyes
Organic synthetic dyes are widely used in various industries including textile, paper, dying, and printing industries due to their inexpensive cost and easy availability- for example, crystal violet, methylene blue, and orange II dyes. The discharge of hazardous contaminants such as different synthetic dyes to waster surfaces makes it unfavourable for living organisms’ usage [134,135,136,137]. The fabric industry sewages contain various sorts of dyes that cause a severe threat to the ecological system [138,139,140]. Furthermore, the adsorption of dye on adsorbent surface is achieved via various adsorption mechanisms, as schematically shown in Fig. 14. It should be noted that the adsorption of water pollutants on adsorbents is mainly guided by electrostatic attraction, π–π interactions, van der Waals forces, hydrogen bonding, acid–base reactions, and hydrophobic interaction [141].
Adsorption processes and mechanisms for dye removal from bulk liquid [141].
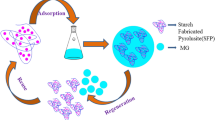
Recently, Farag et al., have been reported radiation technique for grafting AMPS and DMAEMA onto starch for removal of basic dye (Astrazon Red 6B) from aqueous media. The maximal adsorption capacity reached 600 mg/g at pH 9 via electrostatic and H-bonding interactions [55]. While effective disposal of MG dye using potato starch grafted AA/AM hydrogel in the presence of MBA as crosslinker was studied at 60 mg/l of dye. The findings showed good adsorption efficiency of 94.2% at 120 min. The high removal percentage is due to the electrostatically interaction between the negative charged –CONH2, –COO− and –COOH functional groups and positively function groups of MG [142]. Lately, Guo et al., [143], have reported cross-linked starch hydrogels by quaternary ammonium terminals for adsorption of golden yellow (GY) dye. The Qmax from Langmuir model was recorded 208.77 mg/g (99.59%) at 308.15 K, and 14.3 min [143]. A novel hydrogel beads based on gelatin and oxidised maize starch were examined by Dai et al., [144] and the utility and viability of using them to treat wastewater with colours was investigated.. Tartrazine was presented as a model substance. The data illustrated Qmax of 293 mg/g at 35 °C, a pH of 2.5, and stirring at 400 rpm via electrostatic and H-bonding interactions. Mahmoodi et al., [145] designed ethylenediamine/GA-modified starch (SEG/ positive charge) hydrogel for capture DR23 and Acid Blue 92 (AB92) dyes by adsorption technique. The adsorption capacity was growing from 8 mg/g (DR23) to 167 mg/g (AB92) via electrostatic interaction and H-bonding interaction. In another study, Loghin et al. [146], synthesised beads made from chitosan and potato starch, and PAN for removal of CR and DB15 from an aqueous solution. The prepared starch beads achieved good adsorption for CR with 170 mg/g and 75 mg/g for DB15.
Starch-based modified nanocomposites adsorbents are widely applied for removal of different dyes from contaminated water. Starch-g-PAM/GO/HAP nanocomposite adsorbent was prepared by Hossein et al. for removal of MG from an aqueous water. to be environmentally benign and effective at removing various cationic dyes from contaminated water. The Qmax for MG dye was 297 mg/g at pH 10, and the adsorption isotherm data was well fitted with the Langmuir isotherm model. After five consecutive reusable cycles, the obtained nanocomposite demonstrated good regeneration capability without any reduction in adsorption efficiency [26]. According to Pourjavadi1 et al. prepared CaCO3 /starch/PAM/PAMPS/GO nanocomposite adsorbent for detection of MB dye. The adsorption data demonstrated high adsorption capacity of up to 714.29 mg/g at pH 7. The high adsorption capacity of the nanocomposites as a result of presence of different functional groups as Sulfonate, hydroxyl and amide which facilitate the interaction between nanocomposites and MB via electrostatic interaction, hydrogen bonding interactions as well as π-π interactions [147].
Ismail et al. reported that poly(p-aminophenol) (PpAP), containing starch and GO nanocomposite was prepared via free radical polymerization to remove MB dye from an aqueous solution. The fabricated nanocomposite adsorbent was able to remove MB dye by efficiency of 96.7% from water at pH 7. The numerous functional groups contained in the ternary composite, which promote electrostatic reactions, hydrogen bonding, and π-π interactions between the MB dye and the ternary composite. After six regenerated cycles, the synthesised adsorbent also shown good reusability without any decrease in adsorption efficiency of MB dye [148]. Moreover, recently, Hossein zadeh et al., reported the preparation of starch-g-PAM/GO nanosheets / n-HAP nanocomposite (Fig. 15a) for removal of MG dye. The MG dye adsorption maximum was recorded as 297 mg/g at basic pH solution (pH10). After 5 regenerated cycles, the synthesised adsorbent also shown good reusability without any decrease in adsorption efficiency of MG dye (Fig. 15b) [149].
a Schematic preparation of starch-g-PAM/GO nanosheets / n-HAP nanocomposite adsorbent for removal of MG dye, and b five adsorption–desorption cycles for the prepared nanocomposite [149].
On the other hand, starch-based nanoclay nanocomposites have been reported in several literature for removal of dyes. For instance, Lawchoochaisakul et al., mentioned the fabrication of cationic starch/MMT for removal of water-soluble cationic dyes including Basic Blue (BB66) and Basic Yellow (BY1) from solutions. The results showed good ability for the prepared nanocomposite adsorbent with adsorption efficiency up to 99.40% (49.7 mg/g) for BB66 and 99.7% (49.9 mg/g) for BY1, via intercalation of CST into the inter-layer spacing of MMT. The adsorption mechanism showed that the existence of cationic starch may widen the MMT interlayer space, enhancing the adsorption on the surface of the prepared nanocomposite [150].
While the modified starch nanocomposite adsorbents based on metal/metal oxide nanoparticles have been reported for detection of various dyes as well. For instance, recently, El-Sheikh et al., [151], have been reported starch nanocomposite based on carboxy methyl starch copolymerized with PAM/ Ag NPs as an adsorbent for MB from aqueous solutions. The study displayed that removal % of MB reached up to 99.33%, at 343 K [151]. Under batch circumstances, Optilan Blue (OB) adsorption on starch-coated Fe3O4 NPs was carried out. On the adsorption of OB on the prepared nanocomposite, the findings showed that the highst removal of OB wase 88.87% at pH 2 and 308 K [152]. Additionally, Yeamin et al., [153] have reported the preparation of poly (aniline), PANI, /starch/SiO2 nanocomposites for removal of MB and OG dyes via adsorption technique. The MB dye adsorption capacity is 6.8 × 106 mol/g, while the removal capacity of OG was 48 × 106 mol/g at pH 6.86 and 302 K. As a nanofiber membrane for the elimination of MB dye, Moradi et al., [154] fabricated a PVA/starch nanofiber membrane using an electrospinning technique. The findings illustrated that the highest adsorption capacity of MB is 400 mg/g. at pH 8.5, and 150 min. Furthermore, after five reusable cycles, the adsorption capacity remained at 92.1%. Several reported studies in recent years have been mentioned based on different starch formulations for removal of different toxic synthetic dyes (Table 3).
Removal of Antibiotics
Pharmaceutical antibiotics have reported as dangerous water pollutant in recent years [155, 156]. Every year, a massive number of antibiotics are manufactured and consumed. Antibiotics that have not been utilized or digested are discharged into the environment which introduces resistance into natural bacterial ecosystems [157,158,159]. For instance, according to water analysis data, ciprofloxacin and tetracycline have been found at high concentrations in surface water, groundwater, and sediment [160, 161]. Various chemical and physical methods have been used to eliminate organic compounds, including adsorption, biodegradation, chemical oxidation, ion exchange, and membrane approaches [162,163,164,165,166,167]. But the adsorption technique has great attention in few past years due to its outstanding advantages that mentioned above.
Grafted starch has been applied widely for capture of antibiotic drugs from contaminated water. For example, Bouhedda et al. [168] mentioned the designing of an amphiphilic adsorbent system based on grafting of starch with octenyl succinic anhydride for the removal of cephalexin antibiotic with a batch adsorption method. They studied different factors, including octenyl succinic anhydride grafted starch dose, temperature, adsorption time, and pH. Qmax reported as 0.92 mg/g at pH 6, via hydrophobic interaction. The results were well fitted to the Redlich-Peterson isotherm and second-order kinetic model. Most recently, Kang and his co-workers [169], have reported high-efficient modified corn starch-based hyperbranched brush architecture flocculant for removal of tetracycline. They mentioned that the as-prepared flocculant has remarkable advantages, including low-cost material, easy preparation, and eco-friendly. Hyperbranched brush architecture starch-based flocculant has been synthesised by grafting of PAMPS and PAM on the backbone of carboxymethyl corn starch. The obtained flocculant grafted copolymer starch achieved super removal ability for tetracycline reached up to 95.7% via electrostatic and H-bonding interactions.
To remove both tetracycline and ciprofloxacin from wastewater using hydrogel-based adsorbent, Itodo et al. [170] synthesised crosslinked starch/chitosan hydrogel with 2% glutaraldehyde using the casting method for removal of the tetracycline and ciprofloxacin. The results showed that the maximum removal efficiency was 99.4% using the batch adsorption method. The adsorption mechanism was controlled with chemisorption mechanism between adsorbent and adsorbate. On other hand, Fu et al. [171] are interested in preparing a highly efficient starch nanocomposite-based adsorbent for the removal of tetracycline in the presence of nanoscalezerovalent iron using a batch method. The removal efficiency reached up to 99%, which was obtained from the adsorption method (around 30%) and the degradation approach (around 69%) using 200 mg of modified starch and 500 ppm of antibiotic. They reported that both a neutral environment and a high ionic strength enhanced the sedimentation rate. In a recent study for the removal of tetracycline drug, Shen et al. [172] have prepared corn starch and carboxymethyl starch-modified magnetic bentonite clay nanocomposite-based adsorbents. They prepared magnetic bentonite clay with a solvothermal method. The as-prepared carboxymethyl starch/magnetic bentonite showed better adsorption performance (Qmax = 169.7 mg/g) than corn starch nanocomposite (Qmax = 132.3 mg/g) via ion-exchange mechanism, fitting into the Langmuir isotherm and pseudo-second order models. The obtained carboxymethyl starch nanocomposite had good adsorption efficiency and reached up to 59.4% after 3 reused cycles.
Zhang et al. [173] recently have utilized from a biological self-assembly manner for preparation of two starch porous carbon nanocomposites-based adsorbent for the removal of Tetracycline (Fig. 16a). They isolated quinoa starch from quinoa seeds via alkaline steeping technique. They prepared two hyphae/starch composites using fungal hypha of Aspergillusniger and Myrotheciumverrucaria with code number of PCCQS-AN and PCQS-MV, respectively. Surface area of the obtained two composites mentioned as 3050 m2/g and 2546 m2 /g, respectively. The adsorption method of tetracycline illustrated superior adsorption capacity for PCCQS-MV (67.16%) than for PCCQS-AN (57.23%) that occurred through electrostatic, π-π, and H-bonding interactions (Fig. 16b). The regeneration studies showed that the as-obtained composites have the ability to maintained higher efficiency up to 43% after 5 cycles.
a Biological self-assembly manner for preparation of two starch porous carbon nanocomposites, b Adsorption of tetracycline on nanocomposite surface [173].
Additionally Mohamed et al. [174] reported the preparation of Pisumsativum pods biochar (PSPB) NPs / starch nanocomposite for the removal of naproxen drug with mass ratio of 2% (w/w) in the presence of glycerol as crosslinking agent at an elevated temperature. The as-prepared nanocomposites were employed for the removal of naproxen drug using adsorption method. The findings showed excellent adsorption efficiency, reaching up to 309.82 mg/g, fitted to Langmuir isotherm through electrostatic, π-π, and H-bonding interactions. They were able to maintain their efficiency after 10 reusable cycles. Moreover, metal organic frameworks (MOFs) have been considered one of the most attractive nanocomposite formulations in the past few years to capture antibiotic structures from contaminated water. For example, Mohamed and Mahmoud [175] designed starch nanocomposite adsorbent matric onto MOFs which were doped with nanomagnetite to remove fluvastatin antibiotic. They synthesized MOFs based on malonic acid-grafted starch nanocomposites in the presence of zinc acetate/glutaric acid and nanomagnetite (NFe3O4) with microwave irradiation technique according to Fig. 17. The results recorded a high surface area of 528.4 m2/g; mesoporous structure with pore size of 2.9 nm, as well as high crystallinity. The adsorption data for the removal of antibiotic demonstrated a Qmax of 782.1 mg/g via H-bonding interactions, fitting with Langmuir isotherm. The validity of as-prepared nanosorbent for the capture of fluvastatin from real water was proved in the range of 96.2–99.9%.
Schematic preparation of starch nanocomposite adsorbent; NFe3O4@Zn(GA)/malonic acid-grafted starch nanocomposite [175].
In the latter study, Jia and his colleagues reported a hierarchical porous adsorbent for sulfanilamide drug from wastewater [176]. They prepared a porous adsorbent based on starch (maize)/chitosan/UiO-66-COOH-type carboxylic zirconium MOF with a facile Ch-adhesive approach via electrostatic and H-bonding interactions. The results demonstrated that the porous nanocomposite offered a macro-porous structure with high stability. The Qmax recorded to be 46.64 mg/g via electrostatic and H-bonding interactions according to the Langmuir isotherm model, meaning the formation of a homogenous monolayer on the adsorbent surface. And, it was fitted to a pseudo-second-order model. The adsorption mechanism confirmed that the Zr-O moiety in carboxylic Zr-MOF had a high affinity for the sulfanilamide drug. Moreover, the adsorption of the as-obtained adsorbent affected with pH of the antibiotic solution, therefore, it decreased with an increase in pH from 3 to 11. Additionally, the recyclability test showed that the nanocomposite-based adsorbent has the ability to maintain its adsorption efficiency of 30.24 mg/g after 4 reused cycles. Table 3 shows different reported studies in recent years have been mentioned based on different starch formulations for removal of antibiotics.
Removal of Pesticides
Without using pesticides, modern farming is all but impossible. In spite of being first banned or restricted globally in the 1980s, organochlorine pesticides (OCPs) were found to be among the most resilient classes of organic pollutants [177]. Conversely, synthetic pyrethroids and organophosphates are two pesticides that are typically categorised as being substantially less persistent than OCPs and are therefore frequently used in pest management. However, the rising use of synthetic pyrethroids (SPs) and organophosphorus pesticides (OPPs) poses very high chronic and acute toxicity to some aquatic creatures, including fish, invertebrates, and mollusks [178]. Taking into account all of these variables, pesticide use does not ensure water safety. According to literature studies, rivers in agricultural catchments face major difficulties as a result of the pesticides' expanding use at an exponential rate [179, 180]. To date, starch has been highlighted as an attractive natural material for designing high-efficient adsorbent to eliminate pesticides owing to its outstanding advantages, including low cost, biocompatibility, non-toxicity, and easy chemical modification. for example, Tang et al. [181] investigated pesticide removal from basil leaves using gelatinized corn starch (GS) using surface-enhanced Raman scattering (SERS) mapping (Fig. 18). They studied the removal of different pesticide categories, such as organo-phosphorus pesticide like temephos, organo-nitrogen pesticide like acetamiprid, organo-chlorine pesticide like dicofol, and fenvalerate as pyrethroid pesticide. Results showed that the SERS mapping tool is a highly efficient tool to detect pesticide residues on vegetable leaves surface. Moreover, according to the findings, temephos, fenvalerate, dicofol, and acetamiprid can be effectively removed by washing with gelatinized corn starch of 40 g/l for 5 min. The best solution to eliminate pesticide from basil leaves in the absence of “secondary contamination” was gelatinized corn starch.
Pesticide removal from basil leaves using gelatinized corn starch (GS) with SERS mapping [181].
Moreover, Suo et al. [182] reported superior rapid removal of 11 pesticides using mesoporous activated carbon/starch nanocomposites using an adsorption method. The adsorption data of as-prepared starch nanocomposites was recorded to be above 80%, higher than other reported adsorbents such as graphitised carbon black, commercial activated carbon. Findings fitted with the pseudosecond order and Langmuir isotherm models. Moreover, the adsorption mechanism for all tested pesticides enhanced by N atoms, π-π benzene bonds, and oxygen-containing functional groups through hydrophobic interactions and H-bonding interactions. The regeneration test results showed that, after 5 consecutive adsorption–desorption cycles as shown in Fig. 19, the as-obtained starch nanocomposites have good adsorption efficiency, which is still greater than 80% for all pesticides tested.
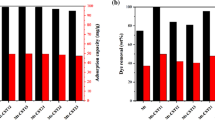
a The adsorption capacity of 11 pesticides by ACS, GCB, AC, C18, and b PSA towards 11 pesticides., Adsorption efficiency of 11 pesticides by ACS after various cycles of regeneration [151].
Removal of Oils
The repercussions of oil contamination on the environment are irreversible. As a result, the removal of oil pollutants has been one of the major environmental issues in the oil and petrochemical industries, where it is necessary to clean contaminated effluents of this contamination [183, 184]. In general, wastewater that has combined with oil in a wide range of quantities is referred to as oily wastewater [185]. In addition to petroleum fractions, the oil molecules detected in oily effluent can also include lipids and hydrocarbons. Absorbents have lately been used as suitable alternatives to clean oil contaminants and waste oils since they are extremely selective, environmentally acceptable, chemically inert, have minimal operational costs as well as considerable absorption capacity, and can be easily recovered [186]. The collection of oily effluent from various industrial sources may differ in terms of its physical properties and chemical content [187].
Currently, a variety of treatment techniques, including adsorption, flocculation, electrocoagulation, and flotation, have been developed for the removal of oil contaminants in order to reduce or prevent the harmful effects of oily wastewater on our ecosystems [188,189,190]. Adsorption has been identified as one of the most promising technology for extracting oil from wastewaters among them since it is one of the least expensive, most efficient methods, and easiest to adapt [190, 191]. Free oil, dispersed oil, emulsified oil, and dissolved oil are the four subcategories of oil-contaminated water, sometimes known as oily wastewater. There are several surface characteristics for effective treatment of oily wastewater. Hydrophobic and oleophilic, hydrophilic and oleophobic, and superhydrophilic and superoleophobic surfaces are among these surface characteristics [192].
According to Wang et al. [193], porous starch-based materials have strong oil absorption capabilities, but their hydrophilicity prevents them from being used to mop up oil spills. By adding nanoparticles of Fe3O4 and silylated SiO2 to starch cryogel, a new superhydrophobic absorbent based on starch designated as (HMS-SiO2@MSC) was created (Fig. 20). The Fe3O4 and silylated SiO2 nanoparticles provided the adsorbent with magnetic (saturation magnetization: 4.36 emu/g) and superhydrophobicity (water contact angle: 154.4°) properties. The (HMS-SiO2@MSC) shown good practicability for cleaning up oil slick on the water's surface using magnets. The benefits of starch and magnetic nanoparticles can be combined in magnetic starch-based adsorbents, which have distinct important characteristics such availability, affordability, size, form, crystallinity, stability, magnetic characteristics, adsorption abilities, and exceptional surface properties. Additionally, the adsorption capacity of HMS-SiO2@MSC toward chloroform and n-hexane is 7.78 and 2.72 g/g respectively.
a, b Schematic synthesis of MSC and HMS-SiO2@MSC, and c adsorption capacity of chloroform and n0hexane by HMS-SiO2@MSC [193].
By hydrolysing hexadecyltrimethoxysilane and tetraethyl orthosilicate in the presence of starch cryogel in a single-step sol–gel procedure, Wang et al. [194] created a superhydrophobic starch-based adsorbent for the removal of oil from water. Starch cryogel is simultaneously given hierarchical micro/nanostructures and low surface energy throughout the process, resulting in a high water contact angle (> 153.0°) and a low sliding angle (< 8.0°). The as-obtained adsorbent has good mechanical and chemical durability, outstanding water repellent, self-cleaning, and anti-fouling capabilities. Furthermore, it demonstrated acceptable oil adsorption performance (2.6–7.5 g/g), which was dominated by its porosity and followed a pseudo-second-order model. Because of its preferential wettability towards water and oil, it was used to remove oil from the water's surface and when submerged. A significant class of smart coatings known as superhydrophobic coatings with large water contact angles (CA > 150) and small water sliding angles (SA 10) have drawn a lot of attention due to their potential applications in a variety of industries, incorporating water/oil separation [195]. Superhydrophobic and magnetic modular starch nanocomposites containing three starch-based modules, namely, a superhydrophobic nano-coating, a magnetic nanocomposite insertion, and a high-strength starch/polyvinyl alcohol composite substrate was prepared by Wang et al. [196] for potential removal of chloroform oil from water. The modular cryogel was exceptionally water-repellent, self-cleaning, and anti-fouling, with high water contact angles (152°) and low slide angles (9°). The adsorption efficiency of the prepared nanocomposite for removal of chloroform reported as 99.8%. After 25 reusable cycles, the adsorption efficiency showed that the prepared nanocomposite maintain its efficiency without any change.
Conclusion, Challenges, and Future Prospects
The adsorption technique is considered one of the most essential ones in recent years for wastewater treatment. Recently, natural polymer such as starch has received great attention for the design of high-performance adsorbents to remove various non-biodegradable contaminants from wastewater, owing to its outstanding features: non-toxic, biodegradable, low-cost material, easily available, and biocompatible. However, it has some drawbacks that make it inappropriate to be used as an adsorbent for wastewater treatment without modification, including low adsorption efficiency and low thermal and mechanical stability. This review presented various starch modification-based adsorbents that can be designed in various formulations such as grafted copolymers, hydrogels, nanofibers, aerogels, and polymer nanocomposites. These modifications can be made in the presence of various synthetic polymers such as poly(acrylic acid), poly(acrylonitrile), and others, as well as natural polymers such as chitosan, gelatin, alginate, and others. In addition to different essential nanofillers, there are carbon-based nanomaterials (such as graphene oxide and carbon nanotube), metal and metal oxide nanoparticles (like Ag NPs and Fe3O4), and nanoclay (such as bentonite and montmorillonite).
The above-mentioned modifications can overcome the limitations of starch to enhance its adsorption ability for wastewater treatment. In addition to enhancing mechanical characteristics, thermal stability, and removal efficiency. Therefore, the review showed the ability of the above-mentioned starch modifications to achieve excellent adsorption efficiency against different non-biodegradable pollutants such as toxic heavy metal ions (for example, Pb2+, Cu2+, Ni2+, Hg2+, and Cd2+ ions), synthetic dyes (such as rhodamine B, crystal violet, malachite green, astrazon red 6B, reactive orange), pharmaceutical antibiotics (for instance, cephalexin, tetracycline, ciprofloxacin, fluvastatin, and sulfanilamide), pesticides (such as temephos, fenvalerate, dicofol, and acetamiprid), and oils and organic solvents (including motor oil, chloroform, hexane, and toluene). Furthermore, the adsorption results for most adsorbed pollutants were fitted to the Langmuir and Freundlich models and a pseudo-second-order kinetic model.
Although starch-based adsorbents have exhibited enormous potency to be employed in water purification, still numerous barriers limit their mercantile applications. The key challenges and recommendations for future work are highlighted below.
-
There is a restricted literature on the use of starch based adsorbents in the real wastewater systems. It is crucial to assess the potential of these adsorbents under realistic circumstance and to evaluate the impact of other pollutants on the sorption potential and selectivity of manufactured adsorbents.
-
Researchers should likewise recognize new strategies to consolidate the adsorption capacity, stability, restoration potential and selectivity of starch-based adsorbents.
-
It is recommended to execute the cost analysis and economic viability to obtain judgment about the sustainable nature of the water treatment process utilizing these adsorbents.
-
Development and synthesis of porous starch using enzymolisation of starch or other methods to enhance its adsorption capacity needs to be inspected in the future.
-
Irradiating starch can be used with other modification strategies, advancing the development of green technology and the economy. This would result in some synergistic effect by decreasing the amount of the agents used in starch modulation.
-
Reusability and renovation of the modified starches are the controlling factors in respect of cost reduction for any industrial and environmental application. Hence adequate selection of desorbing media exists a defiance for the future scientists and environmentalists.
-
Future research should take into account other natural starch supplies, environmentally friendly nanomaterial synthesis, recyclable materials, and the toxicity of nano-waste. For use in commercial applications, further research on biodegradable starch-based hybrids and nanomaterials is required. This research should concentrate on new functional materials, processing innovation, and cost reduction.
-
Future research ought to go into how these adsorbents perform in real water filtration systems with regard to their possible toxicity and environmental impact. Future research should also focus on assessing starch-based composites as potential materials for the manufacturing of membranes for water treatment processes.
References
Gopinath KP, Vo D-VN, Gnana Prakash D, Adithya Joseph A, Viswanathan S, Arun J (2021) Environmental applications of carbon-based materials: a review. Environ Chem Lett 19(1):557–582
Sharma S, Dutta V, Raizada P, Hosseini-Bandegharaei A, Singh P, Nguyen V-H (2021) Tailoring cadmium sulfide-based photocatalytic nanomaterials for water decontamination: a review. Environ Chem Lett 19(1):271–306
Siddiqui SI, Chaudhry SA (2019) Nanohybrid composite Fe2O3-ZrO2/BC for inhibiting the growth of bacteria and adsorptive removal of arsenic and dyes from water. J Clean Prod 223:849–868
Ihsanullah I, Jamal A, Ilyas M, Zubair M, Khan G, Atieh MA (2020) Bioremediation of dyes: Current status and prospects. J Water Process Eng 38:101680
Shivaraju HP, Yashas SR, Harini R (2020) Application of Mg-doped TiO2 coated buoyant clay hollow-spheres for photodegradation of organic pollutants in wastewater. Mater Today Proc 27:1369–1374
Peng H, Guo J (2020) Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: a review. Environ Chem Lett 18(6):2055–2068
Ochedi FO, Liu D, Yu J, Hussain A, Liu Y (2021) Photocatalytic, electrocatalytic and photoelectrocatalytic conversion of carbon dioxide: a review. Environ Chem Lett 19(2):941–967
Matlock MM, Howerton BS, Atwood DA (2002) Chemical precipitation of heavy metals from acid mine drainage. Water Res 36(19):4757–4764
Wang Q, Yu J, Chen X, Du D, Wu R, Qu G, Guo X, Jia H, Wang T (2019) Non-thermal plasma oxidation of Cu (II)-EDTA and simultaneous Cu (II) elimination by chemical precipitation. Environ Manage 248:109237
Wu T, Liu C, Kong B, Sun J, Gong Y, Liu K, Xie J, Pei A, Cui Y (2019) Amidoxime-functionalized macroporous carbon self-refreshed electrode materials for rapid and high-capacity removal of heavy metal from water. ACS Cent Sci 5(4):719–726
Hoseinian FS, Rezai B, Kowsari E, Safari M (2020) A hybrid neural network/genetic algorithm to predict Zn (II) removal by ion flotation. Sep Sci Technol 55(6):1197–1206
Saleem H, Pal P, Haija MA, Banat F (2019) Regeneration and reuse of bio-surfactant to produce colloidal gas aphrons for heavy metal ions removal using single and multistage cascade flotation. J Clean Prod 217:493–502
L. Liu, X.-B. Luo, L. Ding, S.-L. Luo, Application of nanotechnology in the removal of heavy metal from water. In Nanomaterials for the removal of pollutants and resource reutilization. Elsevier, 2019, pp. 83–147
Sun Y, Zhou S, Pan S-Y, Zhu S, Yu Y, Zheng H (2020) Performance evaluation and optimization of flocculation process for removing heavy metal. Chem Eng J 385:123911
Zhang Q, Ye X, Li H, Chen D, Xiao W, Zhao S, Xiong R, Li J (2020) Cumulative effects of pyrolysis temperature and process on properties, chemical speciation, and environmental risks of heavy metals in magnetic biochar derived from coagulation-flocculation sludge of swine wastewater. J Environ Chem Eng 8(6):104472
Maćczak P, Kaczmarek H, Ziegler-Borowska MJM (2020) Recent achievements in polymer bio-based flocculants for water treatment. Material 13(18):3951
Chai WS, Cheun JY, Kumar PS, Mubashir M, Majeed Z, Banat F, Ho S-H, Show P (2021) A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J Cleaner Prod. 296:126589
Akpor OB, Ohiobor GO, Olaolu TD (2014) Heavy metal pollutants in wastewater effluents: sources, effects and remediation. Adv Biosci Bioeng 2(4):37–43
Sanz JL, Köchling T (2007) Molecular biology techniques used in wastewater treatment: an overview. Process Biochem 42(2):119–133
Fang K, Deng L, Yin J, Yang T, Li J, He W (2022) Recent advances in starch-based magnetic adsorbents for the removal of contaminants from wastewater: A review. Int J Biol macromol 218:909–929
Zubair M, Jarrah N, Khalid A, Manzar MS, Kazeem TS, Al-Harthi MA (2018) Starch-NiFe-layered double hydroxide composites: efficient removal of methyl orange from aqueous phase 249:254–264
Elella MHA, Goda ES, Abdallah HM, Abdel-Aziz MM, Gamal H (2023) Green engineering of TMC-CMS nanoparticles decorated graphene sheets for targeting M. tuberculosis. Carbohydr Polym 303:120443
Mittal H, Kumar V, Ray SS (2016) Adsorption of methyl violet from aqueous solution using gum xanthan/Fe3O4 based nanocomposite hydrogel. Int J Biol Macromol 89:1–11
Elmehbad NY, Mohamed NA, Abd El-Ghany NA, Abdel-Aziz MM (2022) Green synthesis of nano-silver/sodium alginate/carboxymethyl xanthan gum hydrogel and evaluation of its anti-inflammatory and anti-helicobacter pylori activity. Cellul Chem Technol 56(9–10):983–995
Abd El-Ghany NA, Mahmoud ZM (2021) Synthesis, characterization and swelling behavior of high-performance antimicrobial amphoteric hydrogels from corn starch. Polym Bull 78:6161–6182
Phan D-N, Khan MQ, Nguyen N-T, Phan T-T, Ullah A, Khatri M, Kien NN, Kim I-S (2021) A review on the fabrication of several carbohydrate polymers into nanofibrous structures using electrospinning for removal of metal ions and dyes. Carbohyd Polym 252:117175
Xie L, Shen M, Wang Z, Xie J (2021) Structure, function and food applications of carboxymethylated polysaccharides: A comprehensive review. Trend Food Sci Technol 118:539–557
Abdallah HM, Elella MHA, Abdel-Aziz MM (2023) One-pot green synthesis of chitosan biguanidine nanoparticles for targeting M. tuberculosis. Int J Biol Macromol 232:123394
Massironi A, Morelli A, Puppi D, Chiellini F (2020) Renewable polysaccharides micro/nanostructures for food and cosmetic applications. Molecules 25(21):4886
Pourjavadi A, Abedin-Moghanaki A, Tavakoli A (2016) Efficient removal of cationic dyes using a new magnetic nanocomposite based on starch-g-poly (vinylalcohol) and functionalized with sulfate groups. RSC Adv 6(44):38042–38051
Haq F, Mehmood S, Haroon M, Kiran M, Waseem K, Aziz T, Farid A (2022) Role of starch based materials as a bio-sorbents for the removal of dyes and heavy metals from wastewater 30(5):1730–1748
Gupta AD, Rawat K, Bhadauria V, Singh HJCP (2021) Recent trends in the application of modified starch in the adsorption of heavy metals from water: a review 269:117763
Nawaz H, Waheed R, Nawaz M, Shahwar D (2020) Physical and chemical modifications in starch structure and reactivity. Cheml Prop Starch. https://doi.org/10.5772/intechopen.88870
Mary SK, Koshy RR, Arunima R, Thomas S, Pothen LA (2022) A review of recent advances in starch-based materials: Bionanocomposites, pH sensitive films, aerogels and carbon dots. Carbohyd Polym Technol App 3:100190
Apriyanto A, Compart J, Fettke J (2022) A review of starch, a unique biopolymer–Structure, metabolism and in planta modifications. Plant Sci 318:111223
Wang S, Chao C, Huang S, Yu J (2020) Starch structure, functionality and application in foods. Springer
Zhang D, Mu T, Sun H (2018) Effects of starch from five different botanical sources on the rheological and structural properties of starch–gluten model doughs. Food Res Int 103:156–162
Pedreiro S, Figueirinha A, Silva AS, Ramos F (2021) Bioactive edible films and coatings based in gums and starch: Phenolic enrichment and foods application. Coatings 11(11):1393
T. Cai, H. Lin, Z. Liu, K. Chen, Y. Lin, Y. Xi, K. Chhuond, Starch wastewater treatment technology. In IOP Conference Series: Earth and Environmental Science, IOP Publishing, 2019, p. 022054.
Liu J, Wang X, Yong H, Kan J, Zhang N, Jin C (2018) Preparation, characterization, digestibility and antioxidant activity of quercetin grafted Cynanchum auriculatum starch. Int J Biol Macromol 114:130–136
Liu Q, Li M, Xiong L, Qiu L, Bian X, Sun C, Sun QJFH (2018) Oxidation modification of debranched starch for the preparation of starch nanoparticles with calcium ions 85:86–92
Cazotti JC, Fritz AT, Garcia-Valdez O, Smeets NM, Dubé MA, Cunningham MF (2019) Grafting from starch nanoparticles with synthetic polymers via nitroxide-mediated polymerization. Macromol Rapid Commun 40(10):1800834
Dufresne AJM (2010) Processing of polymer nanocomposites reinforced with polysaccharide nanocrystals 15(6):4111–4128
Ortega-Toro R, Santagata G, d’Ayala GG, Cerruti P, Oliag PT, Boix MAC, Malinconico M (2016) Enhancement of interfacial adhesion between starch and grafted poly (ε-caprolactone). Carbohyd Polym 147:16–27
Garcia-Valdez O, Champagne P, Cunningham M (2018) Graft modification of natural polysaccharides via reversible deactivation radical polymerization 76:151–173
Brinks MK, Studer AJ (2009) Polymer brushes by nitroxide-mediated polymerization 30(13):1043–1057
Hadjichristidis N, Pitsikalis M, Iatrou H, Driva P, Chatzichristidi M, Sakellariou G, Lohse D (2010). Graft Copolymers. https://doi.org/10.1002/0471440264.pst150.pub2
Sadeghi, G.M.M., Sayaf, M. Nanostructure formation in block copolymers. In Nanostructured polymer blends. Elsevier, 2014, pp. 195-271.
Nemțanu MR, Brașoveanu M, Pincu E, Meltzer VJM (2022) Water-Soluble starch-based copolymers synthesized by electron beam irradiation: Physicochemical and functional characterization 15(3):1061
Ekebafe LO, Ogbeifun DE, Okieimen FE (2018) Equilibrium, kinetic and thermodynamic studies of lead (II) sorption on hydrolyzed starch graft copolymers. J Polym Environ 26(2):807–818
Ismail H, Irani M, Ahmad ZJ (2013) Starch-based hydrogels: Present status and applications 62(7):411–420
Qamruzzaman M, Ahmed F, Mondal M, Ibrahim H (2021) An overview on starch-based sustainable hydrogels: Potential applications and aspects. J Polym Environ 30:1–32
Luo H, Dong F, Wang Q, Li Y, Xiong YJM (2021) Construction of Porous Starch-Based Hydrogel via Regulating the Ratio of Amylopectin/Amylose for Enhanced Water-Retention 26(13):3999
Sinha V, Chakma SJ (2019) Advances in the preparation of hydrogel for wastewater treatment: A concise review 7(5):103295
Farag AM, Sokker HH, Zayed EM, Eldien FAN, Abd Alrahman NM (2018) Removal of hazardous pollutants using bifunctional hydrogel obtained from modified starch by grafting copolymerization. Int J Biol Macromol 120:2188–2199
Thakur K, Rajhans A, Kandasubramanian B (2019) Starch/PVA hydrogels for oil/water separation. Environ Sci Pollut Res 26(31):32013–32028
Fu Y, Yang C, Zheng Y, Jiang J, Sun Y, Chen F, Hu J (2021) Sulfur crosslinked poly (m-aminothiophenol)/potato starch on mesoporous silica for efficient Hg (II) removal and reutilization of waste adsorbent as a catalyst. J. Mol Liquids 328:115420
Muresan EI, Lutic D, Lisa G, Pui A (2017) Mesoporous aluminosilicate macrospheres obtained by spray gelling technique. J Sol Gel Sci Technol 81(3):934–944
Kumar SV, Bafana AP, Pawar P, Rahman A, Dahoumane SA, Jeffryes C (2018) High conversion synthesis of <10 nm starch-stabilized silver nanoparticles using microwave technology. Sci Rep 8(1):1–10
Loghin DF, Bazarghideanu MM, Vasiliu S, Racovita S, Zaharia M-M, Vasiliu T, Mihai MJG (2022) Hydrogel Beads of Amidoximated Starch and Chitosan as Efficient Sorbents for Inorganic and Organic Compounds 8(9):549
Nguyen TPT, Nguyen DV, Thuc CNH, Bui QB, Perré P, Nguyen D (2022) Valorization of starch nanoparticles on microstructural and physical properties of PLA-starch nanocomposites 139(10):51757
de Freitas AdSM, da Silva APB, Montagna LS, Nogueira IA, Carvalho NK, de Faria VS, Dos Santos NB, Lemes AP (2022) Thermoplastic starch nanocomposites: sources, production and applications–a review. J Biomater Sci Polym 33(7):900–945
Onder A, Kıvanç MR, Durmuş S, Ilgin P, Ozay H, Ozay O (2022) Adsorption of malachite green from aqueous solution using hydroxyethyl starch hydrogel improved by graphene oxide. J Polym Environ 30(7):2928–2942
Hasan AMA, Elsaeed SM, Kamal R, Abdel-Raouf ME-S (2016) Low cost biosorbents based on modified starch iron oxide nanocomposites for selective removal of some heavy metals from aqueous solutions. Adv Mater Lett 7(5):402–409
Mahmoud ME, Nabil GM, Zaki MM, Saleh MM (2019) Starch functionalization of iron oxide by-product from steel industry as a sustainable low cost nanocomposite for removal of divalent toxic metal ions from water. Int J Biol Macromol 137:455–468
Ganesamoorthy R, Vadivel VK, Kumar R, Kushwaha OS, Mamane H (2021) Aerogels for water treatment: A review. J Cleaner Product 329:129713
Maleki H, Hüsing N (2018) Current status, opportunities and challenges in catalytic and photocatalytic applications of aerogels: Environmental protection aspects. Appl Catalys B 221:530–555
Wang H, Gong Y, Wang YJRA (2014) Cellulose-based hydrophobic carbon aerogels as versatile and superior adsorbents for sewage treatment 4(86):45753–45759
Kumar, Y., Saxena, D. (2020) A review on starch aerogel: Application and future trends.
Dogenski M, Navarro-Díaz HJ, de Oliveira JV, Ferreira S (2020) Properties of starch-based aerogels incorporated with agar or microcrystalline cellulose 108:106033
Mohammadi A, Moghaddas J (2020) Mesoporous starch aerogels production as drug delivery matrices: synthesis optimization, ibuprofen loading, and release property. Turk J Chem 44(3):614–633
Xie Z, Zhu J, Bi Y, Ren H, Chen X, Yu H (2020) Nitrogen-doped porous graphene-based aerogels toward efficient heavy metal ion adsorption and supercapacitor applications. Rapid Res Lett 14(1):1900534
Zhu F (2019) Starch based aerogels: Production, properties and applications. Trend Food Sci Technol 89:1–10
Li P, Yang C, Xu X, Miao C, He T, Jiang B, Wu W (2022) Preparation of bio-based aerogel and its adsorption properties for organic dyes. Gels 8(11):755
Chen Y, Dai G, Gao Q (2019) Engineering, Starch nanoparticles–graphene aerogels with high supercapacitor performance and efficient adsorption. ACS Sustain Chem Eng 7(16):14064–14073
Kugarajah V, Ojha AK, Ranjan S, Dasgupta N, Ganesapillai M, Dharmalingam S, Elmoll A, Hosseini SA, Muthulakshmi L, Vijayakumar S (2021) Future applications of electrospun nanofibers in pressure driven water treatment: A brief review and research update. J Environ Chem Eng 9(2):105107
Lalia BS, Kochkodan V, Hashaikeh R, Hilal N (2013) A review on membrane fabrication: Structure, properties and performance relationship. Desalination 326:77–95
Brown TD, Dalton PD, Hutmacher DW (2016) Melt electrospinning today: An opportune time for an emerging polymer process. Progress Polym Sci 56:116–166
Chen H, Huang M, Liu Y, Meng L, Ma M (2020) Functionalized electrospun nanofiber membranes for water treatment: A review. Sci Total Environ 739:139944
Sun Z, Li M, Jin Z, Gong Y, An Q, Tuo X, Guo J (2018) Starch-graft-polyacrylonitrile nanofibers by electrospinning. Int J Biol Macromol 120:2552–2559
Kumar D, Pandey J, Raj V, Kumar P (2017) A review on the modification of polysaccharide through graft copolymerization for various potential applications 11:109
Resende JF, Paulino IMR, Bergamasco R, Vieira MF, Vieira AMS (2022) Hydrogels produced from natural polymers: a review on its use and employment in water treatment. Braz J Chem Eng 40:1–16
Qureshi MA, Nishat N, Jadoun S, Ansari MZ (2020) Polysaccharide based superabsorbent hydrogels and their methods of synthesis: A review. Carbohyd Polym Technol App 1:100014
Crini G, Badot PM (2010) Management, Starch-based biosorbents for dyes in textile wastewater treatment. Int J Environ Manage 12(2–4):129–150
Maleki H (2016) Recent advances in aerogels for environmental remediation applications: A review. Chem Eng J 300:98–118
Zuo L, Zhang Y, Zhang L, Miao Y-E, Fan W, Liu TJM (2015) Polymer/carbon-based hybrid aerogels: preparation, properties and applications. Material 8(10):6806–6848
Gamage A, Punniamoorthy T, Madhujith T (2021) Starch-based hybrid nanomaterials for environmental remediation. IntechOpen
Shubhaneel N, Apurba D, Kumar CP (2018) Corn starch industry wastewater pollution and treatment processes—A review. World 1337:1324
Cabral Pinto MM, da Silva EAF (2019) Heavy metals of Santiago Island (Cape Verde) alluvial deposits: Baseline value maps and human health risk assessment. Int J Environ Public Health 16(1):2
CabralPinto MM, Marinho-Reis P, Almeida A, Pinto E, Neves O, Inácio M, Gerardo B, Freitas S, Simões MR, Dinis P (2019) Links between cognitive status and trace element levels in hair for an environmentally exposed population: A case study in the surroundings of the estarreja industrial area. Int J Environ Public Health 16(22):4560
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda K (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60
Yuan W, Yang N, Li X (2016) Advances in understanding how heavy metal pollution triggers gastric cancer. Biomed Res Int 2016:7825432
Zamora-Ledezma C, Negrete-Bolagay D, Figueroa F, Zamora-Ledezma E, Ni M, Alexis F, Guerrero VH (2021) Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ Technol Innov 22:101504
Kamarudzaman AN, Chay TC, Amir A, Talib SA (2015) Biosorption of Mn (II) ions from aqueous solution by Pleurotus spent mushroom compost in a fixed-bed column. World Conf. Technol Innov Enterprenuership 195:2709–2716
Carolin CF, Kumar PS, Saravanan A, Joshiba GJ, Naushad M (2017) Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J Environ Chem Eng 5(3):2782–2799
Shrestha R, Ban S, Devkota S, Sharma S, Joshi R, Tiwari AP, Kim HY, Joshi M (2021) Technological trends in heavy metals removal from industrial wastewater: A review. J Environ Chem Eng 9(4):105688
Palani G, Arputhalatha A, Kannan K, Lakkaboyana SK, Hanafiah MM, Kumar V, Marella RK (2021) Current trends in the application of nanomaterials for the removal of pollutants from industrial wastewater treatment—a review. Molecules 26(9):2799
Panigrahi T, Santhoskumar AU (2020) Adsorption process for reducing heavy metals in textile industrial effluent with low cost adsorbents. Prog. Chem. Biochem. Res. 3(2):135–139
Elella MHA, Aamer N, Mohamed Y, Nazer HAE, Mohamed R (2022) High-potential removal of copper (II) ions from aqueous solution using antimicrobial crosslinked grafted gelatin hydrogels. J Polym Environ 31:1–19
Mgombezi D, Vegi M (2020) An investigation on effectiveness of grafted potato starch as an adsorbent for hard water treatment. J Chem. https://doi.org/10.1155/2020/4050862
Ibrahim B, Fakhre N (2019) Crown ether modification of starch for adsorption of heavy metals from synthetic wastewater. Int J Biol Macromol 123:70–80
Chen Y, Zhao W, Wang H, Meng X, Zhang L (2018) A novel polyamine-type starch/glycidyl methacrylate copolymer for adsorption of Pb (II), Cu (II), Cd (II) and Cr (III) ions from aqueous solutions. R Soc Open Sci 5(6):180281
Aniagor CO, Afifi MA, Hashem A (2021) Heavy metal adsorptive application of hydrolyzed corn starch. J Polym Res 28(11):1–10
Duquette D, Dumont M (2018) Influence of chain structures of starch on water absorption and copper binding of starch-graft-itaconic acid hydrogels. Starch Starke 70(7–8):1700271
Schmidt B, Rokicka J, Janik J, Wilpiszewska K (2020) Preparation and characterization of potato starch copolymers with a high natural polymer content for the removal of Cu (II) and Fe (III) from solutions. Polymers 12(11):2562
Rahman M, Halim M, Deb A, Ahmed S, Dafader N, Alam S, Khandaker S, Alam M (2022) Modification of superabsorbent hydrogels for industrial wastewater treatment. Adv Polym Technol. https://doi.org/10.1155/2022/8405230
Anghel N, Marius N, Spiridon I (2019) Heavy metal adsorption ability of a new composite material based on starch strengthened with chemically modified cellulose. Polym Adv Technol 30(6):1453–1460
Dragan ES, ApopeiLoghin DF, Cocarta AI (2014) Efficient sorption of Cu2+ by composite chelating sorbents based on potato starch-graft-polyamidoxime embedded in chitosan beads. ACS Appl Mater Interfaces 6(19):16577–16592
Chen R, Zhang Y, Shen L, Wang X, Chen J, Ma A, Jiang W (2015) Lead (II) and methylene blue removal using a fully biodegradable hydrogel based on starch immobilized humic acid. Chem Eng J 268:348–355
Dragan ES, Loghin DFA (2018) Fabrication and characterization of composite cryobeads based on chitosan and starches-g-PAN as efficient and reusable biosorbents for removal of Cu2+, Ni2+, and Co2+ ions. Int J Biol Macromol 120:1872–1883
Na Y, Lee J, Lee SH, Kumar P, Kim JH, Patel R (2020) Removal of heavy metals by polysaccharide: A review. Polym-Plast Technol Mater 59(16):1770–1790
Saberi A, Sadeghi M, Alipour E (2020) Design of AgNPs-base starch/PEG-poly (acrylic acid) hydrogel for removal of mercury (II). J Polym Environ 28(3):906–917
Mahmoud ME, Abouelanwar ME, Mahmoud SEM, Salam MA (2021) Doping starch-gelatin mixed hydrogels with magnetic spinel ferrite@ biochar@ molybdenum oxide as a highly efficient nanocomposite for removal of lead (II) ions. J Environ Chem Eng 9(6):106682
Saberi A, Alipour E, Sadeghi M (2019) Superabsorbent magnetic Fe3O4-based starch-poly (acrylic acid) nanocomposite hydrogel for efficient removal of dyes and heavy metal ions from water. J Polym Res 26(12):1–14
Bisht G, Neupane S (2018) Arsenic removal through supercritical carbon dioxide-assisted modified magnetic starch (starch–Fe3O4) nanoparticles. Nanotechnol Environ Eng 3(1):1–14
Xie X, Zhao X, Luo X, Su T, Zhang Y, Qin Z, Ji H (2021) Mechanically activated starch magnetic microspheres for Cd (II) adsorption from aqueous solution. Chin J Chem Eng 33:40–49
Ahamad T, Naushad M, Mousa RH, Alshehri SM (2020) Fabrication of starch-salicylaldehyde based polymer nanocomposite (PNC) for the removal of pollutants from contaminated water. Int J Biol Macromol 165:2731–2738
Chen H, Xu F, Chen Z, Jiang O, Gustave W, Tang X (2020) Arsenic and cadmium removal from water by a calcium-modified and starch-stabilized ferromanganese binary oxide. J Environ Sci 96:186–193
Xu F, Chen H, Dai Y, Wu S, Tang X (2019) Arsenic adsorption and removal by a new starch stabilized ferromanganese binary oxide in water. J Environ Manage 245:160–167
Li P, Gao B, Li A, Yang H (2020) Evaluation of the selective adsorption of silica-sand/anionized-starch composite for removal of dyes and Cupper (II) from their aqueous mixtures. Int J Biol Macromol 149:1285–1293
Yu H, Zhang B, Bulin C, Li R, Xing R (2016) High-efficient synthesis of graphene oxide based on improved hummers method. Sci Rep 6(1):1–7
Kumarathilaka P, Jayaweera V, Wijesekara H, Kottegoda I, Rosa S, Vithanage M (2016) Insights into starch coated nanozero valent iron-graphene composite for Cr (VI) removal from aqueous medium. J Nanomater 2016:1–10
Hosseinzadeh H, Ramin S (2016) Fast and enhanced removal of mercury from aqueous solutions by magnetic starch-g-poly (acryl amide)/graphene oxide nanocomposite superabsorbents. Polym Sci, Ser B 58(4):457–473
Wang Z, Zhang X, Wu X, Yu J-G, Jiang X-Y, Wu Z-L, Hao X (2017) Soluble starch functionalized graphene oxide as an efficient adsorbent for aqueous removal of Cd (II): The adsorption thermodynamic, kinetics and isotherms. J Sol-Gel Sci Technol 82(2):440–449
Bulin C, Zhang B, Guo T, Ma Z, Li B, Zhang Y, Xing R, Ge X (2021) Graphene oxide–starch composite as an efficient adsorbent for removing Cu (II): removal performance and adsorption mechanism. Res Chem Intermed 47(9):3825–3852
Guo T, Bulin C, Zhang B, Ma Z, Xing R, Ge X, Zhang Y, Li B (2022) A highly efficient adsorbent based on starch-graphene oxide architecture for scavenging aqueous Pb (II): isotherms, kinetics, thermodynamics and interaction mechanism. J Polym Environ 30(2):569–584
Beevi BS, Rose KB, Shelvi KA, Kumar MA, Mathew R (2021) Synthesis of starch-graphene oxide composite membrane for heavy metal removal. IOP Conf Ser: Mater Sci Eng 1114:012086
Baghbadorani NB, Behzad T, Etesami N, Heidarian P (2019) Removal of Cu2+ ions by cellulose nanofibers-assisted starch-g-poly (acrylic acid) superadsorbent hydrogels. Compos B Eng 176:107084
Mohamed AK, Mahmoud M (2020) Nanoscale Pisum sativum pods biochar encapsulated starch hydrogel: a novel nanosorbent for efficient chromium (VI) ions and naproxen drug removal. Bioresour Technol 308:123263
Shabtai IA, Lynch LM, Mishael YG (2021) Designing clay-polymer nanocomposite sorbents for water treatment: A review and meta-analysis of the past decade. Water Res 188:116571
Yu C, Tang X, Liu S, Yang Y, Shen X, Gao C (2018) Laponite crosslinked starch/polyvinyl alcohol hydrogels by freezing/thawing process and studying their cadmium ion absorption 117:1–6
Garcia-Padilla A, Moreno-Sader KA, Realpe A, Acevedo-Morantes M, Soares J (2020) Pharmacy Evaluation of adsorption capacities of nanocomposites prepared from bean starch and montmorillonite 17:100292
Camani PH, Dominic CM, Parra DF, Maltez HF, Rosa D (2023) Divalent metal ion removal from simulated water using sustainable starch aerogels: Effect of crosslinking agent concentration and sorption conditions. 226:628–645
Sharma MNG, Kumar A, Rana S, Sharma S, Bhatnagar A, Stadler FJ, Ghfar AA, Khan MR (2017) Process Saf Environ Prot 109:301–310
Elgamal AM, Abd El-Ghany NA, Saad G (2023) Synthesis and characterization of hydrogel-based magnetite nanocomposite adsorbents for the potential removal of acid orange 10 dye and Cr (VI) ions from aqueous solution. Int J Biol Macromol 227:27–44
Elmehbad NY, Mohamed NA, Abd El-Ghany N (2022) Evaluation of the antimicrobial and anti-biofilm activity of novel salicylhydrazido chitosan derivatives impregnated with titanium dioxide nanoparticles. Int J Biol Macromol 205:719–730
Elella MHA, Aamer N, Mohamed YM, El Nazer HA, Mohamed R (2022) Innovation of high-performance adsorbent based on modified gelatin for wastewater treatment. Polym Bull 12:1–17
Ngwabebhoh MGFA, Oladipo AA (2016) Adsorptive removal of multi-azo dye from aqueous phase using a semi-IPN superabsorbent chitosan-starch hydrogel. Chem Eng Res Des 112:274–288
Elgamal AM, Elella MHA, Saad GR, Abd El-Ghany N (2022) Synthesis, characterization and swelling behavior of high-performance antimicrobial biocompatible copolymer based on carboxymethyl xanthan. Mater Today. https://doi.org/10.1016/j.mtcomm.2022.104209
Elgamal AM, Abd El-Ghany NA, Saad G (2022) Highly reactive adsorbent based on carboxymethyl xanthan gum-g-poly (4-vinylpyridine) copolymer for the potential removal of acid orange 10 dye and Cr (VI) ions for water treatment. Appl Polym 139(47):e53179
Dutta S, Gupta B, Srivastava SK, Gupta AK (2021) Recent advances on the removal of dyes from wastewater using various adsorbents: a critical review. Mater Adv 2(14):4497–4531
Al-Aidy H, Amdeha E (2020) Green adsorbents based on polyacrylic acid-acrylamide grafted starch hydrogels: The new approach for enhanced adsorption of malachite green dye from aqueous solution. Int J Environ Anal Chem 101(15):2796–2816
Guo J, Wang J, Zheng G, Jiang X (2019) Optimization of the removal of reactive golden yellow SNE dye by cross-linked cationic starch and its adsorption properties. J Eng Fibers Fabr 14:155892501986526
Dai R, Woo MW, Chen H, Dang X, Mansouri S, Shan Z (2017) Hydrogel beads based on oxidized corn starch cross-linked with gelatin for tartrazine adsorption from aqueous environments. Polym J 49(7):549–555
Mahmoodi NM, Roudaki MSMA, Didehban K, Saeb MR (2019) Ethylenediamine/glutaraldehyde-modified starch: A bioplatform for removal of anionic dyes from wastewater. Korean J Chem Eng 36(9):1421–1431
Loghin DF, Bazarghideanu MM, Vasiliu S, Racovita S, Zaharia M-M, Vasiliu T, Mihai M (2022) Hydrogel beads of amidoximated starch and chitosan as efficient sorbents for inorganic and organic compounds. Gels 8(9):549
Pourjavadi A, Nazari M, Kabiri B, Hosseini SH, Bennett C (2016) Preparation of porous graphene oxide/hydrogel nanocomposites and their ability for efficient adsorption of methylene blue. RSC Adv 6(13):10430–10437
Ismail HK, Ali LIA, Alesary HF, Nile BK, Barton S (2022) Synthesis of a poly(p-aminophenol)/starch/graphene oxide ternary nanocomposite for removal of methylene blue dye from aqueous solution. J Polym Res 29(5):159
Hosseinzadeh H, Ramin S (2018) Fabrication of starch-graft-poly(acrylamide)/graphene oxide/hydroxyapatite nanocomposite hydrogel adsorbent for removal of malachite green dye from aqueous solution. Int J Biol Macromol 106:101–115
Lawchoochaisakul S, Monvisade P, Siriphannon P (2021) Cationic starch intercalated montmorillonite nanocomposites as natural based adsorbent for dye removal. Carbohyd Polym 253:117230
El-Sheikh MA, Almulhim G, Alenazi H (2020) Spectrophotometric studies on the removal of some metal ions and methylene blue dye from their aqueous solutions using new absorbents. Egypt J Chem 63(9):3401–3408
Stan M, Lung I, Soran ML, Opris O, Leostean C, Popa A, Copaciu F, Lazar MD, Kacso I, Silipas TD, Porav AS (2019) Data on the removal of optilan blue dye from aqueous media using starch-coated green synthesized magnetite nanoparticles. Data Brief 25:104165
Yeamin MB, Islam MM, Chowdhury A-N, Awual MR (2021) Efficient encapsulation of toxic dyes from wastewater using several biodegradable natural polymers and their composites. J Clean Prod 291:125920
Moradi E, Ebrahimzadeh H, Mehrani Z, Asgharinezhad AA (2019) The efficient removal of methylene blue from water samples using three-dimensional poly (vinyl alcohol)/starch nanofiber membrane as a green nanosorbent. Environ Sci Pollut Res 26(34):35071–35081
Hoekstra AY (2014) Water scarcity challenges to business. Nature Clim Change 4(5):318–320
Pendergast, M., Hoek, E. (2011) Energy Environ Sci.
Ahmed M, Hameed BJE, Safety E (2018) Removal of emerging pharmaceutical contaminants by adsorption in a fixed-bed column: A review 149:257–266
Baquero F, Martínez J-L, Cantón R (2008) Antibiotics and antibiotic resistance in water environments 19(3):260–265
Kümmerer KJC (2009) Antibiotics in the aquatic environment–a review–part I. Chemosphere 75(4):417–434
Zhu H, Chen T, Liu J, Li D (2018) Adsorption of tetracycline antibiotics from an aqueous solution onto graphene oxide/calcium alginate composite fibers. RSC Adv 8(5):2616–2621
Gani KM, Kazmi AA (2017) Contamination of emerging contaminants in Indian aquatic sources: First overview of the situation. J Hazard Toxic Radioactive Waste 21(3):04016026
Chen W-R, Huang C-HJC (2010) Adsorption and transformation of tetracycline antibiotics with aluminum oxide 79(8):779–785
Choi K-J, Son H-J, Kim S-H (2007) Ionic treatment for removal of sulfonamide and tetracycline classes of antibiotic. Sci Total Environ 387(1–3):247–256
Koyuncu I, Arikan OA, Wiesner MR, Rice C (2008) Removal of hormones and antibiotics by nanofiltration membranes. J Membrane Sci 309(1–2):94–101
Arslan-Alaton I, Dogruel S (2004) Pre-treatment of penicillin formulation effluent by advanced oxidation processes. J Hazard Mater 112(1–2):105–113
Chelliapan S, Wilby T, Sallis P (2006) Performance of an up-flow anaerobic stage reactor (UASR) in the treatment of pharmaceutical wastewater containing macrolide antibiotics. Water Res 40(3):507–516
Mingxuan Y, Jie M, Yiran S, Xinzhu X, Chenlu L, Qiang L, Junhong C (2014) Synthesis of carbon nanotubes/fes fenton-like catalyst and its catalytic properties. Chem J Chin Univ 35(3):570–575
Bouhedda M, Lefnaoui S, Rebouh S, Yahoum MMJC, Systems IL (2019) Predictive model based on Adaptive Neuro-Fuzzy Inference System for estimation of Cephalexin adsorption on the Octenyl Succinic Anhydride starch 193:103843
Kang S, Liu W, Wang Y, Wang Y, Wu S, Chen S, Yan B, Lan XJ (2022) Starch-derived flocculant with hyperbranched brush architecture for effectively flocculating organic dyes, heavy metals and antibiotics. J Taiwan Inst Chem Eng 135:104383
Itodo A, Eneji I, Weor T (2018) Chitosan-starch polymeric blend hydrogels as scavengers of antibiotics from simulated effluent: sorbent characterization and sorption kinetic studies. Adv Polym Sci Technol Int 43(4):22–27
Fu Y, Peng L, Zeng Q, Yang Y, Song H, Shao J, Liu S, Gu J (2015) High efficient removal of tetracycline from solution by degradation and flocculation with nanoscale zerovalent iron. Chem Eng J 270:631–640
Shen Q, Xu M-H, Wu T, Pan G-X, Tang P-S (2022) Adsorption behavior of tetracycline on carboxymethyl starch grafted magnetic bentonite. Chem Pap 76(1):123–135
Zhang B, Jin Y, Huang X, Tang S, Chen H, Su Y, Yu X, Chen S, Chen G (2022) Biological self-assembled hyphae/starch porous carbon composites for removal of organic pollutants from water. Chem Eng J 450:138264
Mohamed AK, Mahmoud ME (2020) Nanoscale Pisum sativum pods biochar encapsulated starch hydrogel: a novel nanosorbent for efficient chromium (VI) ions and naproxen drug removal. Biores Technol 308:123263
Mohamed AK, Mahmoud M (2020) Encapsulation of starch hydrogel and doping nanomagnetite onto metal-organic frameworks for efficient removal of fluvastatin antibiotic from water. Carbohyd Polym 245:116438
Jia X, Zhang B, Chen C, Fu X, Huang Q (2021) Immobilization of chitosan grafted carboxylic Zr-MOF to porous starch for sulfanilamide adsorption. Carbohyd Polym 253:117305
Grung M, Lin Y, Zhang H, Steen AO, Huang J, Zhang G, Larssen T (2015) Pesticide levels and environmental risk in aquatic environments in China—A review. Environ Int 81:87–97
Li H, Cheng F, Wei Y, Lydy MJ, You J (2017) Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: an overview. J Hazard Mater 324:258–271
McKnight US, Rasmussen JJ, Kronvang B, Binning PJ, Bjerg P (2015) Sources, occurrence and predicted aquatic impact of legacy and contemporary pesticides in streams. Environ Pollut 200:64–76
Masiá A, Campo J, Navarro-Ortega A, Barceló D, Picó Y (2015) Pesticide monitoring in the basin of Llobregat River (Catalonia, Spain) and comparison with historical data. Sci Total Environ 503:58–68
Tang J, Zhang Q, Zhou J, Fang H, Yang H, Wang FJFC (2021) Investigation of pesticide residue removal effect of gelatinized starch using surface-enhanced Raman scattering mapping 365:130448
Suo F, Liu X, Li C, Yuan M, Zhang B, Wang J, Ma Y, Lai Z, Ji M (2019) Mesoporous activated carbon from starch for superior rapid pesticides removal. Int j Biol Macromol 121:806–813
Ahmed, J., Thakur, A. (2021) Goyal, Industrial wastewater and its toxic effects (2021).
Abu-Thabit NY, Uwaezuoke OJ, Elella M (2022) Superhydrophobic nanohybrid sponges for separation of oil/water mixtures. Chemosphere 294:133644
Gilbert L, Nwachukwu M, Uzoije A (2018) A review of petroleum waste management and environmental quality status of Niger Delta. Adv Envi Was Mana Rec 1(1):8
Abuhasel K, Kchaou M, Alquraish M, Munusamy Y, Jeng YT (2021) Oily wastewater treatment: Overview of conventional and modern methods, challenges, and future opportunities. Water 13(7):980
Medeiros, A.D.L.M.d., Silva Junior, C.J.G.d., Amorim, J.D.P.d., Durval, I.J.B., Dosta, A.F.d.S., Sarubbo, L.A. Oily wastewater treatment: methods, challenges, and trends. Processes 10(4) (2022) 743.
El-Gawad A (2014) Oil and grease removal from industrial wastewater using new utility approach. Adv Environ Chem. https://doi.org/10.1155/2014/916878
Olajire AA (2020) Recent advances on the treatment technology of oil and gas produced water for sustainable energy industry-mechanistic aspects and process chemistry perspectives. Chem Eng J Adv 4:100049
Azeez RA (2019) Removal oil from produced water by using adsorption method with adsorbent a Papyrus reeds. Eng Technol J 37:157–165
Miranda MA, Ghosh A, Mahmodi G, Xie S, Shaw M, Kim S, Krzmarzick MJ, Lampert DJ, Aichele CP (2022) Treatment and recovery of high-value elements from produced water. Water 14(6):880
Nasrollahzadeh M, Sajjadi M, Iravani S, Varma RS (2021) Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: A review. Carbohyd Polym 251:116986
Wang F, Ma R, Tian Y (2022) Superhydrophobic starch-based nanocomposite cryogel for oil removal underwater and magnetically guided oil slick cleanup. Carbohyd Polym 287:119297
Wang F, Ma R, Tian Y (2022) Fabrication of superhydrophobic/oleophilic starch cryogel via a simple sol-gel immersion process for removing oil from water. Ind Crops Prod 184:115010
Zhang Y, Zhou S, Lv Z, Fan L, Huang Y, Liu X (2022) A facile method to prepare superhydrophobic coatings for various substrates. Appl Sci 12(3):1240
Wang F, Ma R, Zhan J, Tian Y (2022) Superhydrophobic modular cryogel with variable magnetic-actuated motion direction for discrete small-scale oil spill cleanup. J Hazard Mater 430:128448
Al-Aidy H, Amdeha E (2021) Green adsorbents based on polyacrylic acid-acrylamide grafted starch hydrogels: the new approach for enhanced adsorption of malachite green dye from aqueous solution. Int J Environ Anal Chem 101(15):2796–2816
Gad ES, Owda M, Mousa RY (2020) A novel starch nanoparticle citrate based adsorbent for removing of crystal violet dye from aqueous solution. Egypt J Chem 63(6):2075–2095
Ismail HK, Ali LIA, Alesary HF, Nile BK, Barton S (2022) Synthesis of a poly (p-aminophenol)/starch/graphene oxide ternary nanocomposite for removal of methylene blue dye from aqueous solution. J Polym Res 29(5):1–22
Stan M, Lung I, Soran M-L, Opris O, Leostean C, Popa A, Copaciu F, Lazar MD, Kacso I, Silipas T-D (2019) Starch-coated green synthesized magnetite nanoparticles for removal of textile dye Optilan Blue from aqueous media. J Taiwan Inst Chem Eng 100:65–73
Ali S, Gad E (2020) Investigation of an adsorbent based on novel starch/chitosan nanocomposite in extraction of indigo carmine dye from aqueous solutions. Biointerface Res Appl Chem 10:5556–5563
Wang F, Chang R, Ma R, Tian Y (2021) Eco-friendly and superhydrophobic nano-starch based coatings for self-cleaning application and oil-water separation. Carbohyd Polym 271:118410
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work is self-funded by all authors.
Author information
Authors and Affiliations
Contributions
NAAE-G: Conceptualization, Visualization, Writing—original draft, Writing—review & editing. MHAE: Conceptualization, Visualization, Formal Analysis, Writing—original draft, Writing—review & editing. HMA: Resources, prepared all tables, some figures, Writing& editing. MSM: Resources, prepared all figures, Writing—original draft. MS: Resources, prepared all tables, Writing—original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd El-Ghany, N.A., Elella, M.H.A., Abdallah, H.M. et al. Recent Advances in Various Starch Formulation for Wastewater Purification via Adsorption Technique: A Review. J Polym Environ 31, 2792–2825 (2023). https://doi.org/10.1007/s10924-023-02798-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-023-02798-x